Alpha-Linolenic Acid (ALA) Metabolism: Biochemical Pathways, Health Benefits, and Therapeutic Implications for Biomedical Research
This comprehensive review synthesizes current knowledge on alpha-linolenic acid (ALA), an essential omega-3 fatty acid that must be obtained through dietary sources.
Alpha-Linolenic Acid (ALA) Metabolism: Biochemical Pathways, Health Benefits, and Therapeutic Implications for Biomedical Research
Abstract
This comprehensive review synthesizes current knowledge on alpha-linolenic acid (ALA), an essential omega-3 fatty acid that must be obtained through dietary sources. We explore the complex metabolic pathway of ALA conversion to long-chain polyunsaturated fatty acids like EPA and DHA, a process occurring primarily in the endoplasmic reticulum and peroxisomes that is influenced by significant factors including gender, dose, and health status. The article systematically examines ALA's diverse pharmacological effects, including its anti-metabolic syndrome, anticancer, anti-inflammatory, antioxidant, anti-obesity, and neuroprotective properties, alongside its emerging role in modulating intestinal flora. For researchers and drug development professionals, we critically evaluate methodological approaches for studying ALA metabolism, address key challenges in optimizing its bioavailability and efficacy, and provide comparative analysis of its therapeutic potential relative to other omega-3 fatty acids. The review concludes with future directions for translating ALA research into targeted clinical applications and novel therapeutic strategies.
The Essential Nature and Metabolic Fate of Alpha-Linolenic Acid
Alpha-lipoic acid (ALA) is a potent, endogenous organosulfur compound that serves as an essential cofactor in mitochondrial energy metabolism and represents a critical component of the cellular antioxidant network [1] [2]. Its unique amphiphilic character, which allows for solubility in both aqueous and lipid environments, coupled with its ability to cross the blood-brain barrier, distinguishes it from many other antioxidants and underpins its broad therapeutic potential [1] [3]. This whitepaper details the fundamental structural characteristics of ALA and its natural dietary origins, providing a scientific foundation for understanding its role in human physiology and its application in drug development and clinical research, particularly within the context of oxidative stress and inflammatory conditions [1] [2].
Chemical Structure and Properties
The biological activity of alpha-lipoic acid is intrinsically linked to its distinctive chemical architecture.
Core Structure: ALA is systematically named as 1,2-dithiolane-3-pentanoic acid or 5-(1,2-dithiolan-3-yl)pentanoic acid, reflecting its core structure—a cyclic disulfide (dithiolane ring) appended with a five-carbon carboxylic acid chain [1] [3] [4]. This structure classifies it as a derivative of octanoic acid [1].
Isomeric Forms: ALA possesses a chiral center, leading to two enantiomeric forms. The R-(+)-enantiomer (R-ALA) is the form naturally occurring in biological systems and found covalently bound to conserved lysine residues in enzyme complexes, serving as an essential cofactor [1] [2]. The S-(-)-enantiomer (S-ALA) is not naturally produced in the body. Most synthetic supplements contain a racemic mixture (R-ALA and S-ALA) [3].
The ALA/DHLA Redox Couple: A key functional feature is its reversible redox chemistry. The oxidized form, ALA, can be reduced in vivo to dihydrolipoic acid (DHLA), where the disulfide bond is broken to form two thiol (-SH) groups [1] [2]. The ALA/DHLA pair creates a potent redox couple with a standard reduction potential of -0.32 V, which is central to its antioxidant function [1] [2].
Table 1: Key Physicochemical Properties of Alpha-Lipoic Acid
| Property | Description | Biological Implication |
|---|---|---|
| IUPAC Name | 5-(1,2-dithiolan-3-yl)pentanoic acid [1] | Standardized chemical nomenclature |
| Molecular Formula | C₈Hâ‚â‚„Oâ‚‚Sâ‚‚ [5] | Defines elemental composition |
| Solubility | Amphiphilic (soluble in both lipids and water) [1] [2] | Enables systemic distribution and access to both membrane and cytoplasmic compartments |
| Chirality | One chiral center; exists as R-(+) and S-(-) enantiomers [1] [3] | The R-form is biologically active as a cofactor; S-form is synthetic |
| Redox Pair | ALA (oxidized, disulfide) and DHLA (reduced, dithiol) [1] | Forms a potent, regenerative antioxidant system |
While the body endogenously produces ALA, dietary intake can contribute to systemic levels, though the bioavailability is influenced by several factors.
Primary Food Sources: The highest concentrations of ALA are found in animal tissues with high metabolic activity. Rich sources include heart, liver, and kidney [1] [3]. Among plant-based sources, spinach, broccoli, tomatoes, peas, and Brussels sprouts contain appreciable amounts, with spinach being the richest vegetable source [1] [3].
Bioavailability Considerations: In foods, the natural R-ALA enantiomer is often covalently bound to lysine residues in proteins (as lipoyl-lysine), which can limit its systemic bioavailability upon consumption [3]. Dietary absorption from food sources is generally considered insufficient to significantly elevate bloodstream concentrations, which is why ALA is commonly used in supplemental form for therapeutic purposes [1].
Table 2: Alpha-Lipoic Acid Content in Selected Dietary Sources
| Food Source | Estimated ALA Content | Notes |
|---|---|---|
| Spinach | Highest among vegetables [1] | Leading plant-based source; precise concentration data varies. |
| Broccoli | Good source [1] [3] | A valuable dietary component for ALA intake. |
| Tomatoes | Good source [1] [3] | Commonly consumed vegetable source. |
| Beef Heart/Kidney | High [1] | Animal organs are among the most concentrated natural sources. |
| Red Meat | Present [4] | Muscle tissue contains lower levels than organs. |
Table 3: Key Differences Between Alpha-Lipoic Acid and Alpha-Linolenic Acid
| Feature | Alpha-Lipoic Acid (ALA) | Alpha-Linolenic Acid (ALA/ALA) |
|---|---|---|
| Chemical Class | Organosulfur compound with a dithiolane ring [1] | Polyunsaturated fatty acid (Omega-3) [6] |
| Primary Role | Antioxidant, enzyme cofactor in energy metabolism [1] | Structural membrane component, precursor to EPA and DHA [6] |
| Key Dietary Sources | Spinach, broccoli, organ meats (heart, liver, kidney) [1] [3] | Flaxseeds, chia seeds, walnuts, canola oil [7] |
| Solubility | Amphiphilic [1] | Lipophilic [6] |
Molecular Mechanisms and Signaling Pathways
The ALA/DHLA system exerts its effects through multiple, interconnected biochemical mechanisms, which can be visualized as a coordinated network of direct and indirect actions.
Diagram 1: Multimodal Molecular Mechanisms of ALA/DHLA. The diagram illustrates the interconnected direct antioxidant, indirect antioxidant, and cell signaling actions of the ALA/DHLA redox couple, highlighting its role in neutralizing oxidants, regenerating other antioxidants, and modulating key metabolic and inflammatory pathways.
Detailed Mechanism of Action
Direct Radical Scavenging: The ALA/DHLA system is highly effective at quenching a wide spectrum of reactive oxygen species (ROS). DHLA, in particular, is a powerful scavenger of hydroxyl radicals, peroxyl radicals, and hypochlorous acid [1] [2]. It is noteworthy that neither ALA nor DHLA is highly active against hydrogen peroxide [1].
Metal Chelation: Both forms can chelate redox-active transition metals, preventing them from catalyzing the formation of highly damaging free radicals via Fenton reactions. DHLA chelates Fe³âº, Cd²âº, and Hg²âº, while ALA preferentially binds Cu²âº, Zn²âº, and Pb²⺠[1] [2]. This activity is crucial in contexts like Alzheimer's disease, where metal chelation in the brain can reduce free radical damage [1].
Indirect Antioxidant Effects: ALA/DHLA plays a pivotal role in regenerating the oxidized forms of other critical antioxidants, such as vitamin C (from dehydroascorbate) and vitamin E (from the tocopheroxyl radical), effectively recycling them back to their active states [1] [2] [4]. Furthermore, ALA enhances cellular levels of glutathione (GSH) by acting as a transcriptional inducer of genes involved in its synthesis and by increasing the availability of cysteine, a key precursor [1] [5].
Modulation of Key Signaling Pathways: ALA inhibits the activation of the pro-inflammatory transcription factor NF-κB, thereby reducing the expression of cytokines, chemokines, and adhesion molecules [1] [2]. In glucose metabolism, ALA exhibits insulin-mimetic properties by enhancing insulin receptor (IR) and insulin receptor substrate-1 (IRS-1) phosphorylation, activating the PI3K/Akt pathway, and modulating AMPK activity, culminating in the translocation of GLUT4 glucose transporters to the cell membrane and increased glucose uptake [3].
Experimental Analysis Protocols
To investigate the bioavailability and metabolic effects of ALA in a research setting, well-established in vivo and in vitro protocols are employed.
In Vivo Pharmacokinetic and Efficacy Study (Rodent Model)
This protocol is designed to assess the absorption, distribution, and biochemical efficacy of ALA administration in an animal model.
- Objective: To determine the plasma concentration time profile and target tissue uptake of orally administered ALA, and to evaluate its subsequent effect on tissue antioxidant status and glucose metabolism.
Materials:
- Test Compound: R-(+)-Alpha-lipoic acid (purity ≥98%) or racemic ALA [3].
- Animals: Male Wistar or Sprague-Dawley rats (8-10 weeks old).
- Vehicle: Saline or a minimal volume of DMSO followed by dilution in saline for intravenous administration; for oral gavage, ALA can be suspended in a weak alkaline solution to enhance solubility [2].
- Equipment: HPLC system with UV/electrochemical detector, microcentrifuges, spectrophotometer, surgical tools for tissue collection.
Procedure:
- Administration & Sampling: Rats are fasted for 12 hours with free access to water. ALA is administered via oral gavage at a dose of 50-100 mg/kg. Blood samples (~200 µL) are collected from the tail vein or retro-orbital plexus into heparinized tubes at pre-dose, 0.25, 0.5, 1, 2, 4, and 6 hours post-administration [2].
- Plasma Processing: Blood samples are immediately centrifuged at 4°C (3000 × g for 10 min). Plasma is separated and stabilized with an antioxidant preservative (e.g., metaphosphoric acid) and stored at -80°C until analysis.
- Tissue Collection: At terminal time points (e.g., 1 hour and 4 hours), animals are euthanized. Tissues of interest (liver, brain, kidney, skeletal muscle) are rapidly excised, snap-frozen in liquid nitrogen, and stored at -80°C.
- Bioanalysis: ALA and DHLA concentrations in plasma and tissue homogenates are quantified using a validated HPLC method with UV (333 nm) or electrochemical detection.
- Efficacy Biomarkers: Homogenates of liver and muscle are analyzed for:
- Glutathione Status: Ratio of reduced (GSH) to oxidized (GSSG) glutathione using an enzymatic recycling assay.
- Lipid Peroxidation: Measurement of malondialdehyde (MDA) levels via thiobarbituric acid reactive substances (TBARS) assay.
- Glucose Uptake (in muscle): Assessment of GLUT4 translocation to the plasma membrane via subcellular fractionation and western blotting.
In Vitro Glucose Uptake Assay (Cell Culture)
This protocol measures the direct effect of ALA on stimulating glucose uptake in cultured adipocytes or skeletal muscle cells.
- Objective: To quantify ALA-induced glucose uptake and elucidate the involved signaling pathways in a controlled cell culture system.
Materials:
- Cell Line: 3T3-L1 adipocytes (differentiated) or L6 myotubes.
- Test Compounds: ALA, DHLA, specific inhibitors (e.g., LY294002 for PI3K, SB203580 for p38 MAPK, Compound C for AMPK).
- Radiotracer: 2-Deoxy-D-[1,²H]glucose.
- Buffers: Krebs-Ringer Phosphate HEPES (KRPH) buffer (pH 7.4).
- Equipment: Cell culture incubator, scintillation counter, western blot apparatus.
Procedure:
- Cell Treatment: Differentiated adipocytes or myotubes are serum-starved for 3-6 hours. Cells are pre-treated with or without pathway-specific inhibitors for 1 hour, followed by stimulation with ALA (0.1-1.0 mM) or insulin (positive control) for 30-60 minutes [3].
- Glucose Uptake Measurement: Cells are washed with warm KRPH buffer and incubated with 2-Deoxy-D-[1,²H]glucose (e.g., 0.1 µCi/well) in KRPH for a defined period (e.g., 10 minutes). The reaction is stopped by washing cells three times with ice-cold PBS.
- Lysis and Quantification: Cells are lysed with 0.1% SDS. An aliquot of the lysate is used for scintillation counting to measure radiolabeled glucose incorporation. Another aliquot is used for protein quantification (e.g., BCA assay) to normalize uptake data.
- Pathway Analysis: Parallel wells are used for protein extraction. Signaling pathway activation is analyzed by western blotting for phosphorylated and total levels of proteins such as Akt, p38 MAPK, AMPK, and IR/IRS-1.
Diagram 2: In Vitro Glucose Uptake Assay Workflow. The flowchart outlines the key steps for evaluating ALA-induced glucose uptake in cultured adipocytes or myotubes, including treatment, radiotracer-based measurement, and subsequent signaling pathway analysis.
The Scientist's Toolkit: Key Research Reagents
Table 4: Essential Reagents for Investigating Alpha-Lipoic Acid Biology
| Reagent / Material | Function / Application in Research | Example Use Case |
|---|---|---|
| R-(+)-Alpha-Lipoic Acid (≥98%) | Gold standard for studies; represents the natural, biologically active enantiomer. | Investigating specific cofactor roles and high-potency effects in enzyme assays and cell culture [1] [3]. |
| Racemic (R/S) Alpha-Lipoic Acid | Represents the commonly available supplemental form. | Comparative studies to evaluate the efficacy and metabolism of the synthetic vs. natural form [3]. |
| Dihydrolipoic Acid (DHLA) | The reduced, active form of the antioxidant couple. | Directly studying the potent reducing and radical-scavenging capacity of the reduced form [1] [2]. |
| Pathway Inhibitors (e.g., LY294002, SB203580, Compound C) | Pharmacological tools to dissect signaling mechanisms. | Elucidating the contribution of PI3K, p38 MAPK, and AMPK pathways to ALA-induced glucose uptake [3]. |
| 2-Deoxy-D-[1,³H]glucose | Non-metabolizable radiolabeled glucose analog. | Quantifying the rate of glucose transporter activity and glucose uptake in cell cultures [3]. |
| Antibodies (anti-p-Akt, anti-p-p38, anti-GLUT4) | Detection of protein expression and activation states. | Western blot analysis to confirm activation of insulin signaling and downstream pathways [3]. |
| 4-Methoxychalcone | 4-Methoxychalcone|CAS 959-33-1|Research Chemical | |
| Quercetin 3-gentiobioside | Quercetin|CAS 117-39-5|For Research | High-purity Quercetin flavonol for research use only (RUO). Explore its antioxidant, anti-inflammatory, and anticancer research applications. Not for human consumption. |
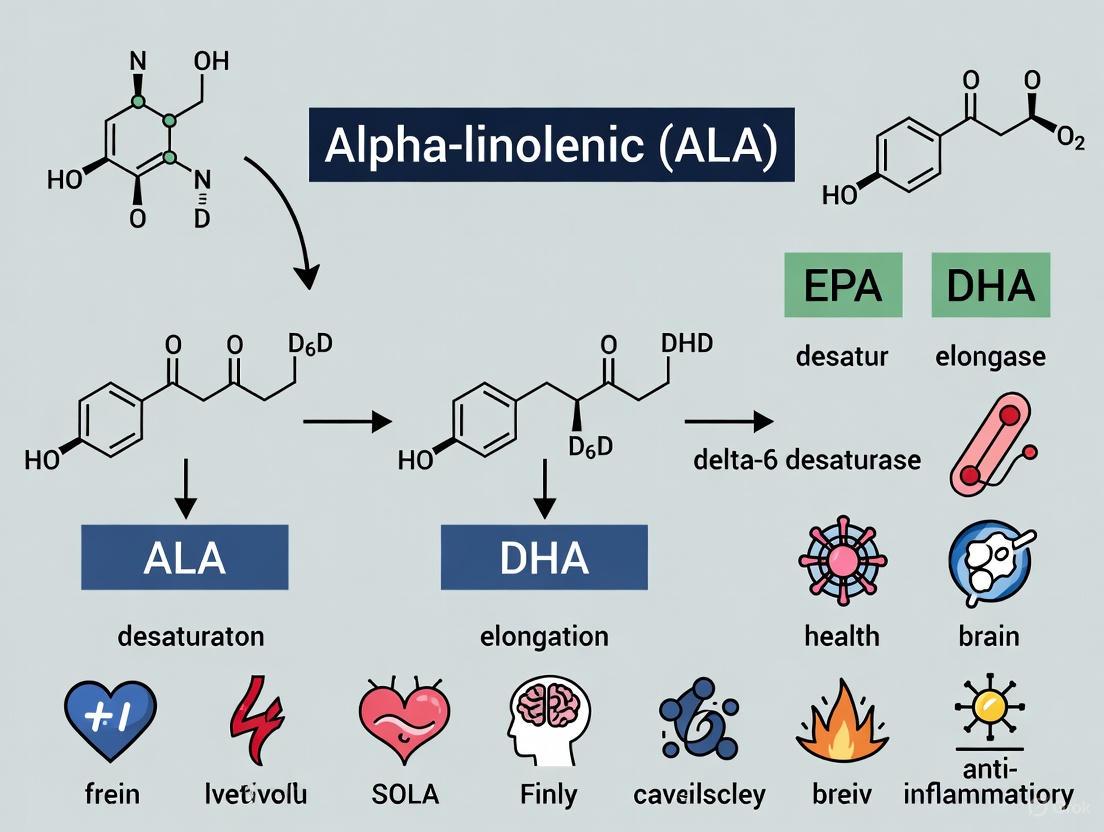
ALA as a Precursor for EPA and DHA Synthesis
Alpha-linolenic acid (ALA) serves as the essential dietary precursor for the synthesis of longer-chain omega-3 polyunsaturated fatty acids (PUFAs), primarily eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). This whitepaper comprehensively examines the metabolic pathways, conversion efficiencies, influencing factors, and methodological approaches for studying ALA metabolism. The conversion process occurs through a series of desaturation and elongation reactions, though this transformation is notably limited in humans, with estimated rates below 8% for EPA and less than 4% for DHA [8]. Recent evidence indicates that sex differences, genetic polymorphisms, dietary composition, and health status significantly impact endogenous synthesis efficiency [9] [8]. Understanding these complex metabolic pathways is crucial for researchers and drug development professionals seeking to optimize omega-3 status through both dietary interventions and targeted therapeutic approaches.
Omega-3 polyunsaturated fatty acids (PUFAs) constitute a family of essential lipids with profound implications for human health. The biochemical classification of PUFAs depends on the position of the terminal double bond relative to the methyl end of the fatty acid chain. Alpha-linolenic acid (ALA), an 18-carbon chain with three double bonds (18:3n-3), represents the essential precursor from which longer-chain omega-3 PUFAs are derived [10]. As an essential fatty acid, ALA cannot be synthesized de novo by humans and must be obtained from dietary sources such as flaxseed, chia, walnuts, and canola oil [11] [10].
The metabolic pathway proceeds through a series of enzymatic reactions involving desaturases and elongases that convert ALA to stearidonic acid (SDA, 18:4n-3), eicosatetraenoic acid (ETA, 20:4n-3), eicosapentaenoic acid (EPA, 20:5n-3), docosapentaenoic acid (DPA, 22:5n-3), and ultimately to docosahexaenoic acid (DHA, 22:6n-3) [12]. The same enzyme systems metabolize both omega-3 and omega-6 fatty acids, creating competitive inhibition between these pathways [13] [8]. The typical Western diet, characterized by a high omega-6 to omega-3 ratio (ranging from 15:1 to 40:1), further limits the already constrained conversion of ALA to EPA and DHA [11]. This metabolic landscape forms the foundation for understanding how dietary ALA contributes to the body's omega-3 status and subsequent health outcomes.
Metabolic Pathways and Conversion Efficiency
The Enzymatic Conversion Pathway
The conversion of ALA to long-chain omega-3 PUFAs involves a coordinated series of enzymatic reactions occurring primarily in the endoplasmic reticulum, with the final step for DHA synthesis taking place in peroxisomes [12]. The pathway begins with rate-limiting delta-6 desaturase (D6D) catalyzing the conversion of ALA to stearidonic acid (SDA). SDA then undergoes elongation to eicosatetraenoic acid (20:4n-3), which is desaturated by delta-5 desaturase (D5D) to form EPA [12] [14].
EPA is elongated to docosapentaenoic acid (DPA, 22:5n-3), which can be further elongated to tetracosapentaenoic acid (24:5n-3). This intermediate then undergoes a second D6 desaturation to tetracosahexaenoic acid (24:6n-3), which is translocated to peroxisomes for beta-oxidation to produce DHA (22:6n-3) [12]. This complex pathway shares the same enzymatic machinery with the omega-6 PUFA metabolism, creating inherent competition between the two pathways based on substrate availability [13].
Figure 1: Metabolic pathway of ALA to EPA and DHA synthesis showing enzymatic steps and cellular compartments.
Quantitative Conversion Efficiency
The conversion efficiency of ALA to long-chain omega-3 PUFAs is fundamentally limited in humans. Recent studies utilizing stable isotope tracers and dietary intervention trials have quantified these conversion rates, revealing significant constraints in the metabolic pathway, particularly for DHA synthesis.
Table 1: Conversion Efficiency of ALA to Long-Chain Omega-3 PUFAs in Humans
| Metabolic Product | Average Conversion Rate | Key Influencing Factors | Research Findings |
|---|---|---|---|
| EPA | <8% [8] | Sex, dietary LA intake, ALA dose | ALA supplementation increases EPA levels in blood and tissues [9] |
| DHA | <4% [8] | Sex, age, genetic polymorphisms | ALA supplementation typically shows no significant effect on DHA levels or omega-3 index [9] |
| Overall Omega-3 Index | Minimal impact from ALA | Baseline omega-3 status, health conditions | EPA and DHA intake considered primary strategy for improving omega-3 index [9] |
The conversion of ALA to EPA and DHA is significantly influenced by dietary factors, particularly the competitive inhibition from high linoleic acid (LA, omega-6) intake [8]. Studies have demonstrated that restricting LA while increasing ALA intake can modestly enhance EPA levels, though the effect on DHA remains limited [8]. Additionally, dose-response relationships have been observed, with one clinical trial finding that 30 grams daily of ground flaxseed successfully raised EPA levels in blood, while 10 grams daily was insufficient [8].
Factors Influencing ALA Conversion Efficiency
Biological and Dietary Determinants
Multiple factors significantly impact the efficiency of ALA conversion to EPA and DHA, creating substantial interindividual variability in endogenous synthesis capacity.
Table 2: Key Factors Influencing ALA Conversion Efficiency
| Factor Category | Specific Factor | Impact on Conversion | Mechanism |
|---|---|---|---|
| Biological | Sex | Premenopausal women show significantly higher conversion, especially to DHA [8] | Estrogen upregulates desaturase enzymes (FADS1, FADS2) [8] |
| Biological | Genetic polymorphisms | FADS gene variants alter desaturase activity and efficiency | Genetic variations affect enzyme expression and function [8] |
| Biological | Health status | Obesity and metabolic diseases reduce conversion efficiency [9] | Altered enzyme activities and inflammatory states impair metabolism [9] |
| Dietary | Omega-6 intake | High LA intake substantially reduces ALA conversion [8] | Competition for shared desaturase and elongase enzymes [13] |
| Dietary | ALA dose | Dose-dependent response observed for EPA synthesis | Threshold effect; sufficient substrate required for measurable conversion [8] |
| Dietary | Pre-formed EPA/DHA intake | Feedback inhibition of conversion pathway | Regulatory mechanisms suppress endogenous synthesis when pre-formed LC-PUFAs are adequate |
Notably, stearidonic acid (SDA), an intermediate in the conversion pathway, demonstrates enhanced conversion to EPA compared to ALA. SDA bypasses the initial rate-limiting delta-6 desaturase step, potentially offering better conversion efficiency for increasing EPA status [14]. However, similar to ALA, SDA conversion to DHA remains substantially limited in most humans [14].
Impact of Obesity and Metabolic Disorders
Recent evidence indicates that obesity and related metabolic diseases significantly alter the conversion efficiency of ALA to long-chain omega-3 PUFAs. The obesity-associated changes in desaturase and elongase activities can impair the metabolic conversion of ALA, potentially creating a vicious cycle of increased metabolic risk [9]. Studies confirm that EPA and DHA intake should be considered as a primary dietary treatment strategy for improving the omega-3 index in obesity and related diseases, as ALA supplementation alone is insufficient to significantly impact DHA status or the omega-3 index in these populations [9].
Experimental Methodologies and Research Approaches
Dietary Assessment and Biomarker Analysis
Research on ALA metabolism employs sophisticated methodological approaches to quantify conversion efficiency and track metabolic fate. Dietary assessment represents the foundational approach, with validated food frequency questionnaires (FFQs) specifically designed to capture habitual intake of ALA and other PUFAs [15]. These instruments have demonstrated strong correlation with blood levels of EPA and DHA (r = 0.62-0.67, p < 0.001) and are particularly effective in identifying individuals with high omega-3 indices (sensitivity 89%, specificity 84%, agreement 86%) [15].
Biomarker analysis provides a more direct assessment of omega-3 status. The omega-3 index, measuring EPA and DHA in erythrocytes, has emerged as a robust biomarker associated with cardiovascular risk [15]. An index of 8% or higher correlates with reduced cardiovascular disease risk, while 4% or lower indicates increased risk [15]. Stable isotope tracer methodologies represent the gold standard for quantifying conversion kinetics, allowing researchers to track the metabolic fate of labeled ALA through the elongation and desaturation pathway to EPA and DHA [12].
Experimental Supplementation Protocols
Clinical trials investigating ALA metabolism typically employ standardized supplementation protocols with precise dosing and duration parameters:
ALA Supplementation Protocol:
- Source: Flaxseed oil, ground flaxseed, or purified ALA
- Dose Range: 1-30 grams ALA daily [8]
- Duration: Typically 8-12 weeks for stabilization of blood levels
- Controls: Placebo or supplemented with other oils (olive, safflower)
- Compliance Measures: Capsule counts, dietary records, biomarker tracking
Blood Collection and Analysis:
- Timing: Fasting blood samples at baseline, 4, 8, and 12 weeks
- Sample Processing: Plasma, erythrocyte, and platelet isolation
- Fatty Acid Analysis: Gas chromatography with flame ionization detection
- Quality Control: Standardized reference materials, duplicate analysis
Research Reagents and Methodological Tools
Table 3: Essential Research Reagents for ALA Metabolism Studies
| Reagent Category | Specific Examples | Research Application | Functional Role |
|---|---|---|---|
| Fatty Acid Standards | Deuterated ALA (d5-ALA), Carbon-13 labeled EPA | Stable isotope tracer studies | Metabolic pathway tracing and kinetic analysis [12] |
| Enzyme Assays | Delta-6 desaturase activity, FADS2 gene expression | Genetic and enzymatic studies | Quantification of rate-limiting conversion steps [12] |
| Chromatography | Gas chromatography (GC), High-performance liquid chromatography (HPLC) | Fatty acid separation and quantification | Precise measurement of fatty acid profiles [15] |
| Cell Culture Models | Hepatocyte cell lines (HepG2), Primary human hepatocytes | In vitro metabolism studies | Controlled environment for pathway analysis [12] |
| Dietary Sources | Flaxseed oil, Echium oil (SDA source), Purified ALA | Supplementation studies | Controlled intervention materials [9] [14] |
| Analytical Kits | Lipid extraction kits, Phospholipid separation kits | Sample preparation and fractionation | Standardized processing of biological samples [15] |
The metabolic conversion of ALA to EPA and DHA represents a complex, multi-step process with significant limitations in humans. While ALA serves as the essential dietary precursor for long-chain omega-3 PUFAs, its conversion efficiency is constrained by enzymatic limitations, competitive inhibition from omega-6 fatty acids, and individual biological factors. The research community has made substantial progress in quantifying conversion rates, identifying influencing factors, and developing sophisticated methodological approaches to study ALA metabolism.
For drug development professionals and researchers, these findings highlight the importance of considering direct EPA and DHA supplementation rather than relying solely on ALA precursors for achieving therapeutic omega-3 status, particularly in specific populations such as those with obesity or metabolic disorders. Future research should focus on optimizing conversion efficiency through dietary modulation, identifying genetic factors influencing desaturase activity, and developing novel delivery systems for enhanced bioavailability of omega-3 fatty acids.
Peroxisomes are single-membrane-bound organelles essential for eukaryotic cellular homeostasis, performing crucial metabolic functions including the β-oxidation of very-long-chain fatty acids (VLCFAs) and the biosynthesis of ether phospholipids [16]. Their role in lipid metabolism is particularly relevant to alpha-linolenic acid (ALA) research, as the final synthesis step of docosahexaenoic acid (DHA), a critical omega-3 derivative of ALA, occurs within peroxisomes [17]. Unlike most organelles of the endomembrane system, peroxisomes possess a unique capacity to import both membrane and matrix proteins directly from the cytosol [18]. However, a subset of their membrane proteins traffics through the endoplasmic reticulum (ER), initiating a multi-step conversion pathway that is fundamental to peroxisome biogenesis and function [18] [19].
Understanding this ER-to-peroxisome pathway is paramount for researchers investigating ALA metabolism and its health benefits. Peroxisomal function directly impacts the conversion efficiency of ALA to its longer-chain, more bioactive metabolites, including eicosapentaenoic acid (EPA) and DHA [17]. Defects in peroxisome biogenesis disrupt this metabolic pathway and are linked to severe neurological disorders, underscoring the organelle's critical role in brain health and lipid homeostasis [20] [16]. This technical guide details the molecular mechanisms of the ER-dependent peroxisomal protein import pathway, providing methodologies and resources to support advanced research in this field.
Core Mechanisms of the ER-to-Peroxisome Pathway
The biogenesis of peroxisomes involves two coordinated mechanisms: de novo generation from the ER and fission from pre-existing organelles [19]. The ER-dependent, or de novo, pathway is responsible for the initial formation of pre-peroxisomal vesicles.
Key Stages ofDe NovoBiogenesis
Vesicle Budding from the ER: The process initiates in the ER membrane with the insertion of key peroxisomal membrane proteins (PMPs). PEX16 is integrated into the ER membrane, where it functions as a docking site for PEX3 [16]. This PEX3-PEX16 complex then recruits other PMPs to form a pre-peroxisomal vesicle that buds from the ER [16] [19].
Formation of Immature Pre-Peroxisomal Vesicles: These ER-derived vesicles are considered "immature" as they contain a basic set of membrane proteins but lack the full complement of matrix enzymes required for metabolic activity [16]. They serve as primordial structures that can mature into functional peroxisomes.
Vesicle Maturation and Fusion: The immature vesicles acquire additional PMPs and matrix proteins through protein import from the cytosol. They may also undergo homotypic fusion (fusion with each other) to form mature, metabolically active peroxisomes [19]. The import of matrix proteins relies on a dedicated translocation machinery composed of peroxins (PEX proteins) that recognize specific targeting signals [18] [16].
Table 1: Key Peroxins in ER-Dependent Peroxisome Biogenesis and Matrix Protein Import
| Peroxin | Primary Function | Process | Human Disease Association |
|---|---|---|---|
| PEX3 | PMP receptor; recruits PEX19; initiates ER-derived vesicle formation [16] | Membrane Biogenesis | Zellweger syndrome spectrum [19] |
| PEX16 | Docking receptor for PEX3 in the ER membrane [16] | Membrane Biogenesis | Zellweger syndrome spectrum [19] |
| PEX19 | Cytosolic chaperone and import receptor for PMPs [16] | Membrane Biogenesis | Zellweger syndrome spectrum [19] |
| PEX5 | Shuttling receptor for PTS1-containing matrix proteins [18] [16] | Matrix Protein Import | Zellweger syndrome spectrum [19] |
| PEX7 | Receptor for PTS2-containing matrix proteins; requires PEX5L in mammals [18] [16] | Matrix Protein Import | Rhizomelic chondrodysplasia punctata [19] |
| PEX11β | Promotes membrane elongation and division; stimulates DRP1 GTPase activity [20] [16] | Proliferation & Fission | Neurodegeneration [20] |
Matrix Protein Import and Pore Formation
Matrix proteins are synthesized on free polyribosomes in the cytosol and contain specific peroxisomal targeting signals (PTS), either PTS1 (C-terminal) or PTS2 (N-terminal) [18] [16]. The PTS1 receptor, PEX5, and the PTS2 receptor, PEX7, deliver their cargo to a common docking complex on the peroxisomal membrane comprising PEX13 and PEX14 [18] [16] [19].
A critical feature of peroxisomal import is its capacity to translocate folded, cofactor-bound, and even oligomeric proteins [18] [19]. The mechanism underlying this capability involves a transient pore. Current models suggest that the cargo-loaded PEX5 receptor integrates into the membrane, oligomerizing with PEX14 to form a transient, gated translocation channel [19]. After cargo release into the matrix, the PEX1-PEX6-PEX26 AAA-ATPase complex, in concert with RING ubiquitin ligases (PEX2, PEX10, PEX12), facilitates the mono-ubiquitination and extraction of PEX5 back to the cytosol for another round of import [16].
Diagram 1: ER to peroxisome biogenesis pathway.
Experimental Protocols for Investigating the Pathway
Protocol 1: Tracking PMP Trafficking from the ER
This protocol utilizes pulse-chase assays and subcellular fractionation to visualize the movement of PMPs from the ER to peroxisomes.
- Primary Objective: To determine the kinetics and pathway of a specific PMP (e.g., PEX3 or PEX16) from its synthesis in the ER to its integration into mature peroxisomes.
- Materials and Reagents:
- Cell Line: Wild-type and PEX-deficient (e.g., ΔPEX3 or ΔPEX19) mammalian fibroblasts [16].
- Radiolabeling Agent: 35S-methionine/cysteine for metabolic pulse-chase labeling.
- Lysis Buffer: Hypotonic buffer for cell disruption.
- Antibodies: Specific antibodies against the target PMP (e.g., anti-PEX3), organelle markers (Calnexin for ER, PMP70 for peroxisomes).
- Density Gradient Medium: Sucrose or iodixanol for equilibrium density centrifugation.
- Methodology:
- Pulse Phase: Incubate cells with 35S-methionine/cysteine for a short period (e.g., 15-30 minutes) to label newly synthesized proteins.
- Chase Phase: Replace radiolabeled medium with complete cold medium and harvest cells at various time points (e.g., 0, 30, 60, 120 minutes).
- Cell Lysis and Fractionation: Lyse cells using a hypotonic buffer. Perform differential centrifugation to obtain a heavy membrane fraction (containing ER, mitochondria, peroxisomes).
- Density Gradient Centrifugation: Resuspend the heavy membrane fraction and load onto a pre-formed sucrose density gradient (e.g., 20-50%). Centrifuge at 100,000 × g for 3 hours to separate organelles based on buoyant density.
- Analysis: Collect gradient fractions. Perform immunoprecipitation with anti-PEX3 antibody. Analyze the radiolabeled PEX3 across fractions by SDS-PAGE and autoradiography. Co-localization with ER and peroxisomal markers confirms the trafficking route.
- Key Controls: Use PEX-deficient cells, which should arrest the process, causing accumulation of the PMP in the ER [16].
Protocol 2:In VitroPeroxisomal Protein Import Assay
This assay reconstitutes the import of matrix proteins into purified peroxisomes, allowing for mechanistic studies.
- Primary Objective: To quantify the import efficiency of PTS1 or PTS2-containing matrix proteins and identify required cytosolic and membrane components.
- Materials and Reagents:
- Purified Peroxisomes: Isolated from rat liver or a model cell line (e.g., HepG2) using density gradient centrifugation.
- Radiolabeled Cargo Protein: 35S-labeled catalase (PTS1) or thiolase (PTS2) synthesized using a rabbit reticulocyte lysate transcription/translation system.
- Energy-Regenerating System: ATP, GTP, creatine phosphate, creatine kinase.
- Protease: Proteinase K to degrade non-imported proteins.
- Protease Inhibitor: PMSF to terminate protease activity.
- Methodology:
- Import Reaction: Combine purified peroxisomes, radiolabeled cargo, cytosolic extract (as a source of soluble peroxins like PEX5), and the energy-regenerating system in import buffer. Incubate at 37°C for 30-60 minutes.
- Protease Protection: Treat the reaction with Proteinase K on ice to digest all proteins outside the peroxisomes. The imported, protease-protected proteins indicate successful translocation across the membrane.
- Termination and Analysis: Stop protease digestion with PMSF. Re-isolate peroxisomes by centrifugation, solubilize, and analyze by SDS-PAGE and autoradiography. The amount of radiolabeled, protease-protected cargo quantifies import.
- Inhibition Studies: Repeat the assay with ATP-depleting systems (apyrase) or antibodies against specific peroxins (e.g., anti-PEX5) to confirm the specificity and requirements of the import process [18].
- Key Parameters: The import of oligomeric proteins can be tested by pre-assembling cargo complexes in the reticulocyte lysate [18].
Table 2: Essential Research Reagents for Studying ER-to-Peroxisome Pathways
| Reagent / Tool | Specific Example | Research Application | Key Function |
|---|---|---|---|
| PEX-Knockout Cell Lines | ΔPEX3, ΔPEX19 human fibroblasts [16] | Establishing genetic requirements for protein trafficking. | Models for peroxisome biogenesis disorders; blocks early biogenesis steps. |
| Organelle-Specific Markers | Anti-Calnexin (ER), Anti-PMP70 (Peroxisomes) [16] | Immunofluorescence and fractionation analysis. | Validates organelle identity and purity in localization studies. |
| PMP Expression Plasmids | Plasmids encoding GFP-PEX3, GFP-PEX16 [16] | Live-cell imaging of de novo biogenesis. | Visualizes the initial stages of pre-peroxisomal vesicle formation from the ER. |
| Recombinant Matrix Proteins | 35S-labeled Catalase (PTS1), Thiolase (PTS2) [18] | In vitro import assays. | Measures import competence and kinetics into isolated peroxisomes. |
| Specific Antibodies vs. Peroxins | Anti-PEX5, Anti-PEX14, Anti-PEX1 [16] [19] | Co-IP, Western Blot, functional inhibition. | Identifies protein complexes and tests functional necessity. |
Integration with ALA Metabolism and Research Implications
The ER-to-peroxisome pathway is not an isolated process; it is functionally coupled with the metabolism of alpha-linolenic acid. The final and critical step in the synthesis of docosahexaenoic acid (DHA) from ALA occurs within peroxisomes via partial β-oxidation [17]. The product of this pathway, DHA, is not only a crucial component of neuronal membranes but also a regulator of peroxisome dynamics itself. DHA has been shown to induce peroxisomal division by augmenting the hyper-oligomerization of PEX11β, thereby promoting the fission of elongated peroxisomes [16]. This creates a feed-forward loop where functional peroxisomes are necessary to produce DHA, which in turn stimulates the proliferation of more peroxisomes.
This interconnection has profound implications for health and disease. Peroxisomal disorders consistently present with severe neurological phenotypes, partly due to impaired synthesis of ether-phospholipids and DHA, both essential for brain structure and function [20] [16]. Furthermore, altered peroxisome dynamics are observed in common neurodegenerative diseases like Alzheimer's and Parkinson's, suggesting a broader role in neuronal health [20] [19]. Recent clinical research also highlights the potential of ALA supplementation, demonstrating that concurrent administration of ALA and L-carnitine significantly reduced migraine frequency and severity and improved mental health outcomes in women [21]. This underscores the translational potential of targeting these metabolic pathways for therapeutic intervention.
Diagram 2: ALA metabolism and peroxisome feedback.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Advanced Research Tools for Mechanistic Studies
| Reagent Category | Product Examples | Specific Research Use | Technical Notes |
|---|---|---|---|
| Live-Cell Organelle Dyes | MitoTracker (mitochondria), ER-Tracker (ER) | Simultaneous staining of multiple organelles for imaging inter-organelle contacts. | Confirm dye compatibility and absence of spectral bleed-through. |
| CRISPR/Cas9 Knock-in Kits | GFP-PTS1 knock-in cell line | Generation of stable cell lines for real-time monitoring of peroxisome abundance and matrix import. | Ideal for high-content screening of compounds affecting peroxisome function. |
| Proximity Ligation Assay (PLA) Kits | Duolink PLA | Detecting and visualizing very close (<40 nm) protein-protein interactions, e.g., between ER and peroxisomal proteins. | Provides superior resolution over standard co-localization microscopy. |
| Recombinant AAA-ATPase Complex | Purified PEX1-PEX6-PEX26 complex [16] | In vitro studies of the receptor recycling step in matrix protein import. | Essential for reconstituting the complete ATP-dependent import cycle. |
| Lipid Standards for Analytics | Deuterated DHA, VLCFA standards | Quantitative mass spectrometry of peroxisomal lipid metabolites (e.g., after ALA supplementation). | Enables precise tracking of metabolic flux through the ALA/DHA pathway. |
| 3,4',7-Trihydroxyflavone | 3,4',7-Trihydroxyflavone, CAS:2034-65-3, MF:C15H10O5, MW:270.24 g/mol | Chemical Reagent | Bench Chemicals |
| Quercetin 7-O-rhamnoside | Vincetoxicoside B | Vincetoxicoside B is a natural flavonoid with research applications in antifungal and antidiabetic studies. It shows synergistic antifungal activity. For Research Use Only. | Bench Chemicals |
The metabolism of alpha-linolenic acid (ALA) to long-chain omega-3 polyunsaturated fatty acids (PUFAs) is a critical biochemical process with profound implications for human health and disease. This transformation is mediated by a series of tightly regulated enzymatic reactions, with fatty acid desaturases (FADS1 and FADS2) and very-long-chain fatty acid elongases (ELOVL) serving as the cornerstone enzymes governing metabolic flux. This technical review comprehensively examines the structural characteristics, functional properties, substrate specificities, and regulatory mechanisms of these key enzymes. We synthesize current evidence on how genetic polymorphisms, epigenetic modifications, and dietary factors modulate enzyme activity and ultimately influence PUFA profiles. The clinical and therapeutic implications of these regulatory mechanisms are discussed, with particular emphasis on the potential for personalized nutrition strategies based on individual genetic makeup. This analysis provides researchers and drug development professionals with a foundational understanding of the molecular machinery driving ALA metabolism and its translational applications.
Alpha-linolenic acid (ALA) serves as the essential omega-3 fatty acid precursor for the biosynthesis of longer-chain, more unsaturated PUFAs, including eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA). These downstream metabolites play vital roles in neuronal development, visual acuity, inflammatory resolution, and cardiovascular protection [22]. The conversion from dietary ALA to these highly bioactive derivatives occurs through a series of alternating desaturation and elongation reactions, with FADS1, FADS2, and ELOVL enzymes constituting the core catalytic machinery [23] [24].
The efficiency of this conversion pathway exhibits significant interindividual variability, influenced substantially by genetic variation in the encoding enzymes [25]. Additionally, emerging evidence indicates that epigenetic mechanisms, including DNA methylation, further modulate gene expression and activity of these enzymes [25]. Understanding the precise molecular mechanisms governing these enzymes is paramount for developing targeted nutritional and therapeutic interventions for metabolic disorders, inflammatory conditions, and other chronic diseases linked to PUFA imbalance.
Enzyme Systems in ALA Metabolism
The metabolic pathway from ALA to DHA involves a coordinated sequence of reactions catalyzed by distinct enzyme families. The desaturation steps are primarily mediated by the FADS enzymes, while the carbon chain elongation is facilitated by the ELOVL family.
Fatty Acid Desaturases (FADS1 and FADS2)
FADS2 (Δ-6 desaturase) initiates the ALA metabolic pathway by catalyzing the conversion of ALA to stearidonic acid (SDA; 18:4n-3). This enzyme also performs the final desaturation step in the "Sprecher pathway" for DHA synthesis, converting 24:5n-3 to 24:6n-3 [24]. FADS1 (Δ-5 desaturase) acts downstream in the pathway, converting eicosatetraenoic acid (20:4n-3) to EPA (20:5n-3) [23].
These enzymes exhibit competitive substrate interactions that significantly impact metabolic outcomes. Notably, ALA dose-dependently inhibits FADS2 conversion of 24:5n-3 to 24:6n-3, creating a critical regulatory node that may explain the observed decrease in DHA levels when dietary ALA intake exceeds certain thresholds [24].
Table 1: Key Characteristics of Fatty Acid Desaturase Enzymes
| Enzyme | Gene | Reaction Catalyzed | Primary Substrates | Key Regulatory Factors |
|---|---|---|---|---|
| FADS2 (Δ-6 Desaturase) | FADS2 | Δ-6 desaturation | ALA (18:3n-3) → SDA (18:4n-3); 24:5n-3 → 24:6n-3 | Genetic polymorphisms (rs174570), DNA methylation, dietary PUFA |
| FADS1 (Δ-5 Desaturase) | FADS1 | Δ-5 desaturation | 20:4n-3 → EPA (20:5n-3) | Genetic polymorphisms, DNA methylation, competitive inhibition |
Very-Long-Chain Fatty Acid Elongases (ELOVL)
The ELOVL family comprises seven enzymes (ELOVL1-7) that catalyze the condensation reaction, the initial and rate-limiting step in fatty acid elongation. These enzymes exhibit distinct substrate specificities that determine their roles in PUFA metabolism [26].
ELOVL2 demonstrates particular importance in the synthesis of DHA, showing preference for C20 and C22 PUFA substrates. This single enzyme catalyzes the sequential elongation of EPA → DPA → 24:5n-3 [24] [27]. The second reaction (DPA → 24:5n-3) appears to saturate at substrate concentrations not saturating for the first reaction (EPA → DPA), potentially creating a metabolic bottleneck that contributes to DPA accumulation [24].
ELOVL5 has broader substrate selectivity, elongating C18-C22 PUFAs. In some species, including chickens, ELOVL5 demonstrates unique capability to efficiently synthesize 24:5n-3, unlike ELOVL5 enzymes from other species [27].
Table 2: Characteristics of ELOVL Enzymes in PUFA Metabolism
| Enzyme | Substrate Chain Length Preference | Specific Role in ALA Metabolism | Tissue Expression Profile |
|---|---|---|---|
| ELOVL2 | C20 and C22 PUFA | EPA → DPA → 24:5n-3; Critical for DHA synthesis | Widespread: brain, liver, adipose tissue [26] |
| ELOVL5 | C18-C22 PUFA | Elongation of intermediate metabolites; Species-specific roles | Highest in testis and epididymis [26] |
| ELOVL1 | C12-C16 fatty acids | Primarily in saturated/monounsaturated VLCFA synthesis | Highly expressed in skin [26] |
Diagram 1: ALA metabolic pathway and regulatory mechanisms. The pathway illustrates the sequential desaturation and elongation steps from ALA to DHA, highlighting key enzymes and regulatory influences including genetic, epigenetic, and dietary factors.
Genetic and Epigenetic Regulation
Genetic Polymorphisms
Genetic variants in the FADS gene cluster represent crucial modifiers of fatty acid metabolism. Single-nucleotide polymorphisms (SNPs) in this region have been strongly associated with altered levels of multiple PUFAs [23] [25]. The rs174570 SNP, located in the FADS1 gene, demonstrates particularly significant effects, with the minor allele associated with decreased FADS1 and FADS2 expression levels [25].
These genetic variations modify the activity of PUFA desaturation and consequently influence lipid composition in human blood and tissues. Importantly, FADS variants have been associated with plasma lipid concentrations, cardiovascular disease risk, overweight, eczema, pregnancy outcomes, and cognitive function [23]. The genotype distribution differs markedly among ethnicities, potentially reflecting evolutionary adaptation to diets with varying PUFA compositions [23].
Epigenetic Mechanisms
Epigenetic regulation, particularly DNA methylation, provides another layer of control over FADS gene expression. Methylation quantitative trait locus (mQTL) analysis of rs174570 has revealed that methylation levels at multiple CpG sites in both FADS1 and FADS2 are strongly associated with this genetic variant [25].
The relationship between methylation and gene expression is complex and site-specific. Methylation levels at three CpG sites in FADS1 were negatively associated with FADS1 and FADS2 expression, while two CpG sites in FADS2 showed positive associations with gene expression [25]. Mediation analysis indicates that the observed effect of rs174570 on gene expression is tightly correlated with the effect predicted through association with methylation, suggesting DNA methylation as a potential mechanistic link between genetic variation and gene expression [25].
Experimental Methodologies and Research Tools
Key Experimental Protocols
Enzyme Activity Characterization: The substrate selectivities, competitive substrate interactions, and dose-response curves of elongase enzymes can be determined after expression in yeast systems. This approach was used to characterize rat Elovl2 and Elovl5, revealing that Elovl2 is active with C20 and C22 PUFAs and catalyzes sequential elongation reactions [24].
Genetic Association Studies: Genome-wide association studies (GWAS) employ rigorous quality control measures for genotyping data, including exclusion of individuals with high missingness, excess autosomal heterozygosity, high relatedness, ambiguous gender, and ancestry outliers. SNP-level quality control typically excludes markers with call rate <98%, significant departures from Hardy-Weinberg equilibrium (P < 1×10^(-6)), and minor allele frequency (MAF) <1% [25].
Fatty Acid Quantification: Comprehensive fatty acid profiling utilizes ultra-performance liquid chromatography quadrupole-time-of-flight mass spectrometry (UPLC-QTOFMS). A panel of 42 free fatty acids (including SFAs, MUFAs, and PUFAs) can be analyzed with quality control measures including reference standard mixtures run after every ten samples. Data processing employs specialized software such as TargetLynx, with manual examination to ensure data quality [25].
DNA Methylation Analysis: Methylation studies require genomic DNA extraction (typically 500 ng) from tissues of interest, followed by bisulfite conversion. Analysis of specific CpG sites in gene regions of interest (e.g., FADS1 and FADS2) provides quantitative methylation data that can be correlated with genetic variants and gene expression [25].
Diagram 2: Experimental workflow for studying genetic and epigenetic regulation of ALA metabolism. The diagram outlines key methodological steps from sample collection through integrated data analysis for comprehensive investigation of fatty acid metabolism regulation.
Research Reagent Solutions
Table 3: Essential Research Reagents and Tools for ALA Metabolism Studies
| Reagent/Tool | Specific Application | Function and Utility |
|---|---|---|
| Infinium Exome-24 BeadChip | Genome-wide genotyping | Interrogates 247,870 SNPs; identifies genetic variants in FADS region [25] |
| UPLC-QTOFMS System | Fatty acid quantification | Precise measurement of 42 individual fatty acids; high sensitivity and resolution [25] |
| RNeasy Plus Universal Kit | RNA isolation from tissues | Maintains RNA integrity for accurate gene expression analysis [25] |
| PrimeScript RT reagent kit | cDNA synthesis | Converts RNA to cDNA with gDNA removal for clean qPCR templates [25] |
| TaqMan Gene Expression Assays | Quantitative PCR | Specific detection of FADS1 (Hs00203685m1) and FADS2 (Hs00927433m1) [25] |
| EpiTect Fast DNA Bisulfite Kit | DNA methylation studies | Converts unmethylated cytosines to uracils while preserving methylated cytosines [25] |
| Yeast Expression System | Enzyme characterization | Functional analysis of elongase substrate specificity and kinetics [24] |
Clinical and Therapeutic Implications
The regulation of ALA metabolism enzymes has significant implications for human health and disease. Discovering differential effects of PUFA supply that depend on variation of FADS genotypes opens opportunities for developing precision nutrition strategies based either on an individual's genotype or on genotype distributions in specific populations [23].
Dysregulated ELOVL expression has been associated with various disease states, including metabolic disorders, skin diseases, neurodegenerative conditions, and cancer [26]. The intricate involvement of ELOVLs in cancer biology, from tumor initiation to metastasis, highlights their potential as targets for anticancer therapies [26].
The interaction between genetic variants and dietary intake represents a crucial consideration for therapeutic interventions. Studies on variations in the FADS gene cluster provided some of the first examples for marked gene-diet interactions in modulating complex phenotypes, such as eczema, asthma, and cognition [23]. This understanding enables more targeted nutritional recommendations based on individual genetic makeup.
FADS1, FADS2, and ELOVL elongases constitute the fundamental enzymatic machinery governing the metabolism of ALA to long-chain PUFAs. Their activity is modulated through complex genetic, epigenetic, and dietary factors that collectively determine an individual's capacity for EPA and DHA synthesis. Understanding the precise molecular mechanisms regulating these enzymes, including the structural characteristics, functional properties, and regulatory networks, provides critical insights for developing targeted interventions for various chronic diseases. Future research directions should focus on further elucidating the tissue-specific regulation of these enzymes, developing isoform-specific modulators, and translating this knowledge into personalized nutrition and therapeutic strategies that optimize PUFA status based on individual genetic and metabolic profiles.
Tissue Distribution and Cellular Incorporation of ALA
Alpha-linolenic acid (ALA, 18:3n-3) is an essential fatty acid that must be obtained from the diet, as the human body lacks the delta-15 desaturase necessary for its synthesis [28]. This carboxylic acid, composed of 18 carbon atoms and three cis double bonds, serves as a crucial metabolic precursor for the synthesis of longer-chain, more unsaturated n-3 fatty acids, particularly eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3) [29] [30]. Understanding the tissue distribution and cellular incorporation of ALA is fundamental to elucidating its health benefits, which include anti-metabolic syndrome effects, anticancer properties, anti-inflammatory actions, antioxidant activity, neuroprotection, and regulation of intestinal flora [29]. This technical review synthesizes current research on ALA metabolism, focusing on the factors influencing its distribution, incorporation, and conversion in biological systems, with particular relevance to drug development and therapeutic applications.
Metabolic Pathways of ALA
Biosynthetic Pathway to Long-Chain Omega-3 Fatty Acids
After ingestion, ALA undergoes a series of metabolic transformations to yield longer-chain, more unsaturated fatty acids through the actions of desaturase and elongase enzymes [28]. The conversion process begins with delta-6 desaturation of ALA to form stearidonic acid (18:4n-3), followed by elongation to eicosatetraenoic acid (20:4n-3), and then delta-5 desaturation to produce EPA (20:5n-3) [31]. Further elongation and desaturation steps eventually yield DHA (22:6n-3) through a process that involves peroxisomal beta-oxidation [28].
This metabolic pathway competes directly with the parallel pathway for the n-6 fatty acid linoleic acid (LA, 18:2n-6), as both families utilize the same set of enzymes (desaturases and elongases) [31] [28]. The high consumption of LA in typical Western diets (with an n-6/n-3 ratio of approximately 10:1) negatively influences EPA and DHA synthesis from ALA [28]. The modern Western-type dietary pattern has been associated with elevated saturated fatty acid and n-6 PUFA intake, coupled with decreased n-3 PUFA intake, creating an imbalance that affects the metabolic fate of ingested ALA [28].
Table 1: Key Enzymes in ALA Metabolic Pathway
| Enzyme | Gene Symbol | Reaction Catalyzed | Cofactors/Requirements |
|---|---|---|---|
| Delta-6 desaturase | FADS2 | Conversion of ALA to Stearidonic Acid (SDA) | NADH, Molecular Oxygen |
| Elongase 2/5 | ELOVL2/ELOVL5 | Elongation of SDA to Eicosatetraenoic Acid | Malonyl-CoA, NADPH |
| Delta-5 desaturase | FADS1 | Conversion of Eicosatetraenoic Acid to EPA | NADH, Molecular Oxygen |
| Beta-oxidation enzymes | Various | Peroxisomal chain shortening to DHA | ATP, Carnitine |
Regulatory Mechanisms and Transcriptional Control
The metabolism of ALA is regulated at multiple levels, including transcriptional control of the enzymes involved in its elongation and desaturation. Key transcription factors such as peroxisome proliferator-activated receptor alpha (PPARα) and sterol regulatory element binding protein 1c (SREBP-1c) regulate expression of delta-5-desaturase (D5D) and delta-6-desaturase (D6D) [31]. These transcription factors are themselves regulated by various kinases, including mitogen-activated protein kinases (MAPK) [31]. Research using human hepatoma cells has demonstrated that the ratio of n6 to n3 fatty acids differentially regulates the transcript levels of genes encoding these enzymes [31].
LCPUFA and their derivatives function as ligands for nuclear transcription factors, including peroxisome proliferator-activated receptors (PPARs), and suppressors of sterol regulatory element binding proteins (SREBP) [31]. PPARα plays a central role in fatty acid homeostasis by regulating the degradation of fatty acids through mitochondrial, peroxisomal, and microsomal fatty acid oxidation pathways [31]. In contrast, SREBP-1 isoforms are more active in regulating hepatic synthesis of fatty acids [31].
Tissue-Specific Distribution Patterns
Hepatic Processing and Distribution
The liver serves as the primary site for ALA metabolism and distribution to peripheral tissues. In human hepatoma cells (HepG2), the conversion of ALA to EPA and DHA has been shown to be highly dependent on the ratio of linoleic acid (LA) to ALA in the incubation medium [31]. Maximum conversion was observed at an LA/ALA ratio of 1:1, where 17% of recovered [13C]ALA was converted to EPA and 0.7% to DHA [31]. This highlights the significant competitive inhibition between n-6 and n-3 fatty acid families for the enzyme systems responsible for their conversion to longer-chain homologs.
The distribution of ALA and its metabolites to peripheral tissues occurs via complex transport mechanisms involving lipoproteins and fatty acid-binding proteins. Following hepatic processing, these fatty acids are incorporated into various lipid classes, particularly triglycerides and phospholipids, and exported to other tissues [28]. The incorporation into phospholipid membranes is especially significant, as it influences membrane fluidity, receptor function, and signal transduction pathways [28].
Tissue Selectivity and Preferential Incorporation
Different tissues exhibit varying capacities for incorporating ALA and its metabolites, with selective retention mechanisms favoring certain long-chain PUFAs. Tissues with high metabolic activity and specialized functions, such as brain, retina, testes, heart, and kidneys, demonstrate particularly high incorporation of LCPUFAs into membrane phospholipids [31]. The constitutive properties of PUFA in biological membranes contribute to fluidity and integrity of membrane bilayer structures [31].
The brain and nervous tissue show exceptional selectivity for DHA, with limited direct uptake of ALA but efficient incorporation of pre-formed DHA [29] [30]. This selective incorporation is mediated by specific fatty acid transport proteins at the blood-brain barrier that preferentially recognize and transport long-chain omega-3 PUFAs. Cardiac tissue also demonstrates preferential incorporation of ALA metabolites, particularly EPA and DHA, which influence cardiac rhythm and contractility.
Table 2: Tissue Distribution of ALA and Metabolites
| Tissue | Primary ALA Metabolites | Incorporation Efficiency | Major Lipid Classes | Special Characteristics |
|---|---|---|---|---|
| Liver | EPA, DPA, DHA | High conversion, lower DHA | Phospholipids, Triglycerides | Primary metabolic site |
| Brain | DHA | Limited ALA, high DHA uptake | Phospholipids | Selective barrier transport |
| Retina | DHA | Very high DHA retention | Phospholipids | Photoreceptor membrane component |
| Adipose | ALA, EPA | High ALA storage | Triglycerides | Long-term storage depot |
| Cardiac | EPA, DHA | Moderate conversion, high uptake | Phospholipids | Electrical stability |
| Plasma | ALA, EPA, DHA | Variable based on intake | Phospholipids, Esters | Short-term pool, transport |
Cellular Incorporation Mechanisms
Membrane Integration and Lipid Raft Organization
At the cellular level, ALA and its metabolites are incorporated into membrane systems through specific enzymatic processes that determine their final localization and functional roles. Once inside cells, fatty acids are activated to acyl-CoAs by acyl-CoA synthetases and then directed to various metabolic fates, including incorporation into complex lipids by acyltransferases [28]. The composition of fatty acids in membrane phospholipids influences membrane physical properties, including fluidity, phase behavior, and formation of specialized microdomains known as lipid rafts.
The incorporation of ALA and its metabolites into specific phospholipid classes is regulated by the substrate specificity of lysophospholipid acyltransferases, which show preferences for certain acyl-CoAs. Phosphatidylcholine and phosphatidyl ethanolamine are major reservoirs for polyunsaturated fatty acids in cellular membranes, with different subcellular membranes exhibiting distinct fatty acid compositions that reflect their specialized functions.
Intracellular Trafficking and Metabolic Channeling
Cellular incorporation of ALA involves complex intracellular trafficking mechanisms that direct the fatty acid to specific metabolic fates and subcellular locations. After cellular uptake, ALA can be directed toward mitochondrial or peroxisomal beta-oxidation for energy production, incorporated into storage triglycerides, integrated into membrane phospholipids, or channeled into the elongation/desaturation pathway for conversion to EPA and DHA [30] [28].
The metabolic channeling of ALA toward specific fates is influenced by numerous factors, including nutritional status, hormonal signals, and genetic factors. Insulin, glucagon, thyroid hormones, and other endocrine factors regulate the partitioning of ALA between oxidative and synthetic pathways [32]. The competition between ALA and LA for the same enzymatic machinery creates a metabolic bottleneck at the level of delta-6 desaturase, which has a higher affinity for ALA but is often overwhelmed by the higher concentrations of LA in typical diets [31] [28].
Experimental Models and Methodologies
In Vitro Cell Culture Models
HepG2 human hepatoma cells have been extensively utilized as an in vitro model system to study ALA metabolism and incorporation. The standard experimental protocol involves growing cells in T-75 cell culture flasks in Dulbecco's modified Eagle's medium (DMEM) containing 20% fetal calf serum (FCS), 2% glutamine, and 2% penicillin/streptomycin solution at 37°C in a humidified atmosphere of 95% air/5% CO₂ [31]. For experimentation, cells are transferred to T-25 cell culture flasks at a density of 5 × 10ⶠcells and, after 24 hours, the medium is replaced with serum-free DMEM [31].
After another 24 hours, mixtures of [13C] labeled LA and ALA methyl esters are added to give a final fatty acid concentration of 100 μM, typically suspended by sonication in sterile bovine serum albumin (Fraction V) [31]. Researchers commonly test various ratios of LA/ALA, such as 9:1, 4:1, 1:1, 1:0, and 0:1, with incubation periods of 24 hours at 37°C [31]. Quadruplicate determinations are recommended for statistical reliability. Following exposure, cells are harvested using accutase, washed with FCS-free DMEM, centrifuged at 500 × g, and the cell pellet is suspended in sodium chloride solution (0.9%) for storage at -75°C until analysis [31].
Lipid Extraction and Analytical Methods
Lipid extraction from cells or tissues typically employs a modified Folch extraction method [31]. This procedure involves adding 100 μl of internal standard (1 mg/ml of tetracosanoic acid and 3 mg/ml heptadecanoic acid, dissolved in dichloromethane:methanol, 2:1, v:v) to each sample prior to extraction [31]. The resulting lipid extracts can then be analyzed using various chromatographic and mass spectrometric techniques to quantify ALA and its metabolites.
Gas chromatography with flame ionization detection or mass spectrometry is commonly employed for fatty acid profiling, often following transesterification to fatty acid methyl esters. The use of stable isotopically labeled precursors, such as [13C]ALA, allows for precise tracking of metabolic fluxes through various pathways and distinguishes newly synthesized metabolites from pre-existing pools [31]. Advanced lipidomic approaches using liquid chromatography coupled with mass spectrometry enable comprehensive profiling of multiple lipid species and their molecular compositions.
Table 3: Key Research Reagents for ALA Studies
| Reagent/Category | Specific Examples | Function/Application | Experimental Notes |
|---|---|---|---|
| Cell Lines | HepG2 (human hepatoma) | In vitro metabolism studies | Maintain in DMEM + 20% FCS [31] |
| Isotopic Tracers | [13C]ALA-methylesters | Metabolic flux analysis | Suspend in BSA via sonication [31] |
| Chromatography Standards | Tetracosanoic acid, Heptadecanoic acid | Internal standards for quantification | Add prior to Folch extraction [31] |
| Lipid Extraction Solvents | Dichloromethane:methanol (2:1) | Total lipid extraction | Modified Folch method [31] |
| Enzyme Inhibitors | N-MMP, 2,2'-dipyridyl (DPD) | Hme synthesis inhibition | Concentration optimization required [33] |
| Heme Scavengers | Hemopexin (Hx) | Heme sequestration | 0.4 μg/L effective in cucumber [33] |
Factors Influencing Distribution and Incorporation
Dietary and Nutritional Factors
The efficiency of ALA incorporation into tissues and its conversion to longer-chain metabolites is significantly influenced by dietary composition, particularly the balance between n-6 and n-3 fatty acids. Studies have demonstrated that the ratio of linoleic acid (LA) to ALA powerfully impacts the conversion efficiency, with maximum conversion observed at a 1:1 ratio in hepatoma cells [31]. In human studies, lowering dietary LA intake while maintaining constant ALA intake increased EPA concentrations in plasma phospholipids, indicating enhanced conversion efficiency [31].
The absolute amounts of dietary ALA and LA also influence metabolic outcomes, with some research suggesting that these absolute amounts may be more significant than their ratio alone [31]. Other dietary components, including antioxidants, minerals, and macronutrients, further modulate ALA metabolism and incorporation. Natural antioxidants help protect PUFAs from oxidative degradation, which is particularly important for ALA due to its high susceptibility to oxidation resulting from its three double bonds [28].
Physiological and Genetic Modifiers
Multiple physiological and genetic factors significantly impact ALA distribution and cellular incorporation. Gender differences are particularly notable, with women demonstrating higher conversion of ALA to EPA and DHA compared to men, potentially due to regulatory effects of estrogen [30]. This gender difference may be an important consideration in making dietary recommendations for n-3 PUFA intake and in drug development targeting fatty acid metabolism [30].
Genetic polymorphisms in the fatty acid desaturase (FADS) gene cluster significantly influence ALA metabolism, with certain variants associated with more efficient conversion to long-chain metabolites. Other genetic factors, including polymorphisms in elongase enzymes and transcription factors involved in lipid metabolism, further contribute to individual variations in ALA handling. Age, hormonal status, health conditions, and gut microbiota composition represent additional modifiers that influence the ultimate distribution and metabolic fate of dietary ALA.
Implications for Drug Development and Therapeutics
Pharmaceutical Targeting of ALA Metabolism
The understanding of ALA tissue distribution and cellular incorporation provides valuable insights for pharmaceutical development, particularly for drugs targeting metabolic syndrome, cardiovascular diseases, and inflammatory conditions. The regulatory nodes in ALA metabolism, including transcription factors PPARα and SREBP-1c, represent promising therapeutic targets for modulating fatty acid composition and downstream signaling pathways [31]. Drugs that enhance the conversion of ALA to EPA and DHA or that mimic their effects could provide alternatives to marine-derived omega-3 supplements.
The competition between n-6 and n-3 pathways suggests therapeutic potential in modulating the LA/ALA ratio through either dietary interventions or pharmacological approaches that selectively inhibit n-6 metabolism while promoting n-3 incorporation. The development of formulations that enhance ALA stability and bioavailability, such as micro- and nano-encapsulation, represents another promising avenue for therapeutic applications [28].
Biomarker Development and Personalized Nutrition
Quantitative understanding of ALA distribution and incorporation enables the development of biomarkers for assessing omega-3 status and predicting individual responses to interventions. The fatty acid composition of plasma phospholipids, erythrocyte membranes, and adipose tissue provides insights into long-term omega-3 status and metabolic efficiency [28]. These biomarkers can stratify patients according to their metabolic phenotypes for personalized nutritional and pharmaceutical interventions.
The tissue-specific differences in ALA incorporation highlight the importance of considering target tissues when evaluating therapeutic efficacy. Interventions aimed at neuroprotection may require different formulations and dosing strategies compared to those targeting cardiovascular benefits, reflecting the distinct distribution patterns and metabolic handling of ALA and its derivatives in different tissues. This nuanced understanding facilitates the development of more targeted and effective therapeutic approaches based on the fundamental principles of ALA distribution and metabolism.
The tissue distribution and cellular incorporation of ALA involve complex, highly regulated processes that determine its ultimate biological effects and health benefits. The liver serves as the primary site for ALA metabolism, converting it to longer-chain, more unsaturated fatty acids that are then distributed to peripheral tissues via specific transport mechanisms. Cellular incorporation involves targeted trafficking to various metabolic fates, including beta-oxidation for energy production, storage in lipid droplets, and integration into membrane phospholipids where it influences membrane properties and signaling functions.
Multiple factors modulate these processes, including dietary composition (particularly the n-6/n-3 ratio), genetic polymorphisms, gender, hormonal status, and health conditions. Understanding these modulators provides opportunities for therapeutic interventions aimed at optimizing ALA distribution and metabolism for specific health outcomes. Continued research in this area, particularly utilizing advanced tracer methodologies and omics technologies, will further elucidate the nuances of ALA handling in biological systems and support the development of targeted nutritional and pharmaceutical approaches for chronic disease prevention and management.
Factors Influencing ALA Bioavailability and Absorption
Alpha-lipoic acid (ALA), an endogenous disulfide derivative of octanoic acid, demonstrates significant therapeutic potential for diabetic peripheral neuropathy, oxidative stress management, and metabolic disorders. However, its clinical efficacy is substantially influenced by complex factors affecting its bioavailability and absorption. This technical review comprehensively examines the chemical, biological, and formulation variables that modulate ALA pharmacokinetics, with particular focus on enantiomeric specificity, administration protocols, and advanced delivery systems. The analysis integrates current research findings to provide evidence-based guidance for optimizing ALA bioavailability in research and therapeutic applications, addressing a critical need in the context of ALA metabolism and health benefits research.
Alpha-lipoic acid (1,2-dithiolane-3-pentanoic acid) possesses unique physicochemical properties that directly influence its absorption and metabolic fate [34]. As a sulfur-containing compound that functions as an essential cofactor for mitochondrial enzyme complexes, ALA's bioavailability is challenged by several inherent factors: limited gastrointestinal stability, susceptibility to hepatic degradation, and differential handling of its enantiomeric forms [35] [34]. Understanding these fundamental constraints provides the foundation for developing strategies to enhance its therapeutic application.
The molecular structure of ALA, featuring a dithiolane ring with a chiral center at the C6 position, dictates its biochemical behavior [1]. Endogenously, only the R-(+)-enantiomer (R-ALA) is synthesized and functions as a protein-bound cofactor, whereas most supplemental ALA is produced as a racemic mixture (50/50 ratio of R-ALA and S-ALA) [35]. This distinction is critically important because the enantiomers demonstrate different absorption profiles, with human studies showing maximum plasma concentrations of R-ALA are approximately double those of S-ALA following oral administration of the racemic mixture [34].
Key Determinants of ALA Bioavailability
Enantiomeric Form
The stereospecificity of ALA metabolism significantly impacts its bioavailability. The R-enantiomer represents the biologically active form synthesized endogenously and exhibits superior pharmacokinetic properties compared to the S-form [35]. Clinical pharmacokinetic studies demonstrate that R-ALA achieves higher plasma levels and more effectively elevates tissue antioxidant status due to its specific interaction with cellular transport mechanisms and metabolic enzymes [36].
Table 1: Comparative Pharmacokinetics of ALA Enantiomers
| Parameter | R-ALA | S-ALA | Racemic Mixture | References |
|---|---|---|---|---|
| Relative Bioavailability | High (Endogenous form) | Low (Synthetic form) | Intermediate | [35] [34] |
| Plasma Concentration (Cmax) | High (~2× S-ALA) | Low | Moderate | [34] |
| Cellular Uptake | Efficient via specific transporters | Reduced efficiency | Variable | [35] |
| Mitochondrial Targeting | High affinity | Limited affinity | Moderate | [1] [35] |
| Industrial Production Method | Chemical/ enzymatic resolution | Chemical synthesis | Racemization process | [35] |
Administration Factors
Timing Relative to Meals
Administration protocol significantly influences ALA absorption kinetics. Clinical evidence indicates that ALA absorbs best when taken on an empty stomach – specifically at least 2 hours after eating or 30 minutes before a meal [37]. Concurrent food intake, particularly with high-fat meals, can substantially reduce the absorption rate and maximum plasma concentration, potentially through competition with dietary lipids for transport mechanisms or delayed gastric emptying [37].
Dosage and Dosing Regimen
Oral dosage of alpha-lipoic acid in clinical studies ranges from 200 to 1,800 mg daily, with 600 mg established as the standard dose showing optimal balance of efficacy and tolerability [36] [34]. Higher doses do not proportionally increase bioavailability due to saturation of absorption mechanisms and potentially increased first-pass metabolism. Divided dosing regimens (e.g., 300 mg twice daily) may help maintain more stable plasma concentrations while reducing peak-related gastrointestinal side effects [36].
Formulation Advances
Conventional Formulations
Standard ALA supplements are typically available in capsule or tablet forms containing either racemic ALA or R-ALA [36]. These conventional formulations face challenges including susceptibility to oxidation, variable inter-individual absorption, and significant pre-systemic metabolism [38]. Stability maintenance during storage and transport requires protective packaging and potentially stabilizers to prevent degradation that compromises efficacy [38].
Enhanced Bioavailability Formulations
Recent advances in delivery systems aim to overcome ALA's bioavailability limitations:
- Nanoencapsulation: Emerging technologies for 2025 include nanoencapsulation and sustained-release systems designed to improve bioavailability and patient compliance [38]. These systems protect ALA from degradation and enhance cellular uptake.
- Stabilized R-ALA: Specialized formulations using only the R-enantiomer with stabilization technologies to preserve potency [36]. While showing promise, these formulations currently lack the volume of long-term clinical research supporting racemic ALA [36].
- Liposomal Delivery: Phospholipid-based encapsulation that enhances both solubility and absorption while providing protection from gastric degradation [39].
Table 2: ALA Formulation Technologies and Bioavailability Impact
| Formulation Type | Technology Principle | Bioavailability Relative to Standard | Development Status | References |
|---|---|---|---|---|
| Racemic ALA Capsules | Standard 50/50 R/S mixture | Baseline | Clinically established | [36] [34] |
| Stabilized R-ALA | Selective R-enantiomer with stabilizers | Increased (estimated 30-40%) | Commercial availability | [36] |
| Nanoencapsulated ALA | Polymer nanoparticles | Under investigation | Research phase | [38] |
| Liposomal ALA | Phospholipid bilayer encapsulation | Increased (preliminary data) | Early commercial stage | [39] |
| Sustained-Release | Modified release matrices | Possibly improved trough levels | Development phase | [38] |
Experimental Methodologies for Assessing ALA Bioavailability
Pharmacokinetic Study Protocols
Well-established clinical protocols exist for evaluating ALA bioavailability in human subjects. These methodologies typically employ randomized, crossover designs with precise sampling schedules to characterize absorption kinetics [34].
Standard Protocol:
- Subjects: Healthy volunteers or patient populations (n=12-20 for adequate power)
- Design: Randomized, single-dose crossover with washout period (≥48 hours)
- Administration: 600 mg racemic ALA or equivalent R-ALA dose after overnight fast
- Blood Sampling: Pre-dose (0 h) and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, and 8 hours post-administration
- Sample Analysis: HPLC with UV or MS detection for ALA and DHLA quantification
- Pharmacokinetic Parameters: Cmax, Tmax, AUC0-t, AUC0-∞, t1/2, CL/F
Analytical Methodologies
Accurate quantification of ALA and its reduced form, dihydrolipoic acid (DHLA), requires specialized analytical approaches due to their reactivity and rapid metabolic interconversion.
High-Performance Liquid Chromatography (HPLC) Protocol:
- Apparatus: HPLC system with UV detector or LC-MS/MS for enhanced sensitivity
- Column: C18 reverse-phase column (150 × 4.6 mm, 5 μm)
- Mobile Phase: Acetonitrile:methanol:water (40:15:45 v/v/v) with 0.1% formic acid
- Flow Rate: 1.0 mL/min
- Detection: UV at 332 nm or MS detection in negative ion mode
- Sample Preparation: Plasma protein precipitation with acetonitrile, derivatization for stability
- Quantification: External calibration curve (10-1000 ng/mL range)
This methodology allows simultaneous quantification of both ALA and DHLA, providing comprehensive pharmacokinetic profiling of the redox couple [34].
Metabolic Pathways Influencing ALA Bioavailability
Absorption and Distribution
ALA's amphiphilic nature, conferred by its dual solubility in both aqueous and lipid environments, enables unique distribution characteristics throughout the body, including penetration across the blood-brain barrier [1] [37]. Following absorption, ALA is rapidly taken up by tissues, with the highest concentrations accumulating in liver, heart, and skeletal muscle [34].
Biotransformation and Elimination
The primary metabolic fate of absorbed ALA involves reduction to dihydrolipoic acid (DHLA) via NADH-dependent enzymes, particularly dihydrolipoamide dehydrogenase (DLD) [35]. This reduction occurs rapidly in tissues, creating the powerful ALA/DHLA redox couple that exerts antioxidant effects. Subsequent metabolism involves mitochondrial β-oxidation, with shortening of the carbon side chain to produce bisnorlipoic acid and tetranorlipoic acid as primary metabolites [34].
The reduced bioavailability of the S-enantiomer results from its preferential metabolism and elimination rather than tissue incorporation. Renal excretion clears both parent compound and metabolites, with nearly complete elimination within 24 hours post-administration [34].
Research Reagents and Methodological Toolkit
Table 3: Essential Research Reagents for ALA Bioavailability Studies
| Reagent/Category | Specification | Research Application | Key Suppliers |
|---|---|---|---|
| R-ALA Standard | >99% enantiomeric purity | Reference standard for analytical methods; metabolic studies | Sigma-Aldrich, Cayman Chemical |
| Racemic ALA | 50:50 R/S mixture | Comparative bioavailability studies; clinical trial material | Sigma-Aldrich, Merck Millipore |
| Deuterated ALA | Isotopically labeled (d4-ALA) | Internal standard for LC-MS quantification; tracer studies | Cambridge Isotope Laboratories |
| Dihydrolipoic Acid | Reduced form of ALA | Metabolic pathway analysis; redox studies | Cayman Chemical, Santa Cruz Biotechnology |
| DLD Enzyme | Dihydrolipoamide dehydrogenase | Enzyme kinetics; reduction pathway studies | Sigma-Aldrich, Abcam |
| ALA Antibodies | Polyclonal/monoclonal specific to ALA | Immunoassays; tissue localization studies | MyBioSource, Abbexa |
| Caco-2 Cell Line | Human colon adenocarcinoma | In vitro absorption models; transport studies | ATCC, Sigma-Aldrich |
| HPLC Columns | C18 reverse phase (5μm) | Analytical separation of ALA/DHLA | Waters, Agilent, Phenomenex |
| Casticin | Casticin | Bench Chemicals | |
| N-Desmethylgalantamine | N-Desmethyl Galanthamine | N-Desmethyl Galanthamine, a key metabolite of galantamine, is for research into acetylcholinesterase inhibition and drug metabolism. For Research Use Only. Not for human use. | Bench Chemicals |
The bioavailability and absorption of alpha-lipoic acid are governed by a complex interplay of enantiomeric specificity, administration conditions, and formulation technology. Current evidence strongly supports the superiority of R-ALA bioavailability, yet racemic mixtures remain clinically relevant due to their extensive safety database and lower production costs. The ongoing development of advanced delivery systems, particularly nanoencapsulation and stabilized enantiomeric formulations, represents the most promising approach to overcoming current bioavailability limitations.
Future research priorities should include standardized bioavailability assessment protocols, head-to-head comparisons of emerging formulation technologies, and population-specific pharmacokinetic studies to guide personalized dosing regimens. As ALA continues to demonstrate therapeutic potential across multiple oxidative stress-related conditions, optimizing its bioavailability remains essential for maximizing clinical efficacy.
Analytical Approaches and Pharmacological Applications of ALA
Stable Isotope Tracer Methods for Studying ALA Metabolism
Alpha-linolenic acid (ALA), an essential omega-3 polyunsaturated fatty acid, plays crucial roles in human health through its metabolism to long-chain derivatives and its direct biological effects. Stable isotope tracer methodologies provide powerful tools for investigating the in vivo kinetics, metabolic fate, and health implications of ALA in human subjects. This technical guide comprehensively details the principles, experimental protocols, and analytical techniques for applying stable isotope tracers to ALA metabolism research, with particular emphasis on quantifying metabolic flux, turnover rates, and incorporation into various lipid pools. The approaches outlined herein enable precise, safe investigation of ALA dynamics in human systems, supporting advanced research on its cardioprotective effects, optimal dosing, and relationship with chronic disease risk.
Stable isotope tracers are molecules where one or more atoms have been replaced by a less abundant, stable isotope of the same element (e.g., ^13^C, ^2^H, ^15^N), creating a chemically identical but mass-different version that can be tracked through metabolic pathways [40]. These tracers allow researchers to quantify dynamic metabolic processes in living systems without the safety concerns associated with radioactive isotopes, making them particularly valuable for human studies [41]. The fundamental principle underlying this methodology is that the metabolic system treats the tracer identically to the naturally occurring molecule (tracee), yet the mass difference enables detection and quantification using specialized analytical techniques [42].
The application of stable isotopes to study lipid metabolism represents a significant advancement over traditional concentration measurements alone. While standard biochemical analyses provide static snapshots of metabolite levels, stable isotope tracers enable researchers to determine kinetic parameters such as production rates, conversion efficiencies, half-lives, and turnover rates in various physiological and pathological states [41]. For ALA research specifically, this methodology has been instrumental in establishing its conversion kinetics to eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), tissue-specific uptake, and differential metabolic handling under various conditions [43].
Fundamental Principles of Isotopic Tracers
Stable vs. Radioactive Isotopes
Stable isotopes do not undergo radioactive decay and pose no radiation hazard, making them safe for vulnerable populations including children, pregnant women, and patients with chronic diseases [41]. This safety profile enables longer-term studies that can capture subtle metabolic changes over days or weeks, which is particularly advantageous for investigating ALA incorporation into tissue pools with slow turnover rates [42]. In contrast, radioactive tracers have limited applicability in human research due to safety concerns and ethical restrictions, especially for vulnerable populations and long-term studies.
The most commonly used stable isotopes in metabolic research include ^13^C (carbon-13), ^2^H (deuterium), and ^15^N (nitrogen-15), which can be incorporated into ALA molecules at specific positions to track different metabolic fates [41]. The natural abundance of these isotopes provides a baseline that must be accounted for in tracer studies, with typical natural abundance of ^13^C being approximately 1.1% of total carbon [41].
Tracer Design and Selection
Strategic design of tracer molecules is crucial for obtaining meaningful metabolic information. For ALA research, common labeling strategies include:
- Uniformly labeled ALA: All carbon atoms potentially replaced with ^13^C, useful for overall metabolic fate studies
- Position-specific labeling: ^13^C or ^2^H incorporated at specific carbon positions (e.g., carboxyl end) to track particular metabolic pathways
- Deuterated ALA: Hydrogen atoms replaced with deuterium at specific positions for tracking without significant metabolic isotope effects
The selection of tracer design depends on the specific research question, with position-specific labeling particularly valuable for elucidating enzymatic pathways and uniformly labeled tracers better suited for comprehensive fate mapping [40].
Diagram 1: Stable Isotope Tracer Design Options for ALA Research
Methodological Approaches for ALA Metabolism Studies
Tracer Administration Protocols
The method of tracer administration significantly influences the type of metabolic information that can be obtained. Each approach offers distinct advantages for investigating different aspects of ALA metabolism.
Intravenous infusion provides precise control over tracer delivery and enables rapid achievement of steady-state plasma enrichment, making it ideal for kinetic studies requiring precise rate measurements [41]. This method typically involves a primed, continuous infusion where an initial bolus (prime) is followed by a constant infusion to maintain stable plasma enrichment levels. For ALA studies, this approach allows accurate determination of plasma appearance and disappearance rates, conversion efficiency to longer-chain metabolites, and oxidation rates when combined with breath ^13^CO~2~ measurements [44].
Oral administration more closely mimics dietary intake and provides information on absorption, first-pass metabolism, and portal circulation effects [42]. This approach can utilize either chemically synthesized labeled ALA or intrinsically labeled biological sources. Intrinsically labeled proteins or lipids are produced by administering stable isotope tracers to plants or animals, resulting in naturally incorporated labels in food matrices [42]. For example, flaxseeds grown in ^13^CO~2~ atmosphere produce intrinsically labeled ALA that can be used to study the complete digestive and metabolic handling of dietary ALA from whole food sources.
Deuterium oxide (D~2~O) labeling offers a unique approach for measuring longer-term metabolic integration by leveraging the body's natural hydrogen exchange mechanisms [42]. After oral D~2~O administration, deuterium incorporates into multiple metabolic pools, including the synthesis of new ALA molecules and its longer-chain derivatives. This method is particularly valuable for studying tissue-specific incorporation and turnover rates over days or weeks, capturing chronic adaptive metabolic responses that might be missed in shorter infusion studies [42].
Sample Collection and Processing
Appropriate sample collection and processing are critical for obtaining reliable metabolic data. The specific samples required depend on the research questions but typically include:
Blood samples collected at predetermined time points allow determination of tracer enrichment kinetics in plasma lipid fractions. Processing typically involves lipid extraction followed by thin-layer chromatography or solid-phase extraction to isolate specific lipid classes (phospholipids, triglycerides, cholesteryl esters, non-esterified fatty acids) before further analysis [41].
Tissue biopsies (e.g., adipose, muscle) provide information on tissue-specific uptake and storage. Adipose tissue biopsies are particularly relevant for ALA research due to its role as a significant reservoir for polyunsaturated fatty acids. Sample processing involves lipid extraction followed by purification of specific lipid classes and conversion to derivatives suitable for gas chromatography analysis [42].
Breath samples collected in specialized containers allow determination of ^13^CO~2~ enrichment when using ^13^C-labeled ALA, providing information on complete oxidation. This is typically analyzed by isotope ratio mass spectrometry (IRMS) for high-precision measurement of low enrichment levels [45].
Diagram 2: Experimental Workflow for ALA Tracer Studies
Analytical Techniques for Isotope Measurement
Mass Spectrometry Methodologies
Gas Chromatography-Mass Spectrometry (GC-MS) represents a cornerstone analytical technique for ALA tracer studies due to its robust quantification capabilities and relatively accessible instrumentation [41]. The process involves converting ALA and its metabolites to volatile derivatives (typically fatty acid methyl esters) that can be separated by GC before ionization and mass analysis [41]. GC-MS operated in selected ion monitoring (SIM) mode provides sensitivity for detecting low levels of isotopic enrichment in specific metabolic pools, with typical measurement errors of less than 2% when carefully optimized [44]. Electron impact ionization typically produces fragment ions that can be monitored to determine both the position and extent of labeling, providing information on metabolic pathways.
Liquid Chromatography-Mass Spectrometry (LC-MS) has emerged as a powerful alternative that often eliminates the need for derivatization, simplifying sample preparation [41]. Modern high-resolution LC-MS systems can separate complex lipid mixtures and provide both structural information and isotopic distributions. This technique is particularly valuable for studying intact lipid species and their tracer incorporation patterns without the need for prior hydrolysis and derivatization [42].
Isotope Ratio Mass Spectrometry (IRMS) represents the gold standard for high-precision measurement of isotopic enrichment, capable of detecting natural abundance-level variations with exceptional accuracy [45]. Unlike conventional MS that scans across mass ranges, IRMS uses multiple Faraday cups to simultaneously collect specific ions, providing precise isotope ratio measurements [45]. For ALA metabolism studies, GC-combustion-IRMS provides the highest precision for ^13^C enrichment measurements, which is particularly important when enrichment levels are low or when studying slow metabolic processes such as adipose tissue turnover [42].
Data Interpretation and Kinetic Calculations
The fundamental parameter measured in stable isotope studies is the tracer-to-tracee ratio (TTR), which represents the ratio of labeled to unlabeled molecules in a sample [41]. This is typically calculated by measuring the abundance of the labeled form (M+X) relative to the unlabeled form (M+0) after correcting for natural abundance. The TTR can be converted to mole percent excess (MPE), which represents the percentage of molecules that contain the label above natural abundance levels [41].
For kinetic analysis under steady-state conditions, the rate of appearance (R~a~) of ALA in plasma can be calculated using the isotope dilution principle:
R~a~ = F × (E~infusate~/E~plasma~ - 1)
Where F is the tracer infusion rate, E~infusate~ is the enrichment of the infusate, and E~plasma~ is the steady-state enrichment in plasma [41].
Fractional synthetic rate (FSR) of ALA incorporation into various lipid pools or conversion to longer-chain metabolites can be calculated from the time-dependent increase in product enrichment relative to precursor enrichment:
FSR = (ΔE~product~/Δt) / E~precursor~
Where ΔE~product~/Δt is the slope of product enrichment over time and E~precursor~ is the average precursor enrichment during that period [42].
Table 1: Key Analytical Techniques for ALA Tracer Studies
| Technique | Detection Principle | Sensitivity | Applications in ALA Research | Limitations |
|---|---|---|---|---|
| GC-MS | Electron impact ionization with mass separation | ~0.1 atom percent excess [42] | Kinetic studies, position-specific metabolism, metabolic flux analysis | Requires derivatization; may provide limited structural information |
| LC-MS | Electrospray ionization with mass separation | ~0.01 atom percent excess | Intact lipid analysis, complex lipid mixtures, high-throughput applications | Less established for precise isotope ratio measurements |
| GC-IRMS | Combustion to COâ‚‚ with isotope ratio measurement | ~0.0005 atom percent excess [42] | High-precision kinetic studies, low-enrichment samples, long-term turnover | Requires complete conversion to measured gas; less structural information |
| NMR | Nuclear magnetic resonance of ^13^C nuclei | ~1-5% enrichment | Position-specific metabolism, non-destructive analysis, metabolic pathway mapping | Lower sensitivity; requires higher enrichment levels |
Experimental Design Considerations for ALA Research
Study Population Characteristics
The metabolic handling of ALA varies substantially based on physiological and pathological factors that must be considered in experimental design. Age significantly influences ALA metabolism, with studies demonstrating altered conversion efficiency to EPA and DHA in older populations, potentially contributing to age-related inflammatory states [42]. Sex differences in ALA metabolism have been documented, with females often showing higher conversion rates possibly related to hormonal influences on desaturase enzymes [43]. Genetic polymorphisms in fatty acid desaturase (FADS) genes create substantial interindividual variability in ALA conversion efficiency, suggesting potential benefits for genotyping participants in ALA tracer studies [43].
Health status profoundly affects ALA metabolism, with conditions such as metabolic syndrome, diabetes, and inflammatory states altering both ALA kinetics and metabolic fate [43]. For studies focusing on ALA's health benefits, careful participant selection and stratification are essential for obtaining meaningful results. The safety profile of stable isotopes enables inclusion of vulnerable populations who might derive particular benefit from ALA, including those with existing cardiovascular disease, diabetes, or other metabolic disorders [41].
Isotope Delivery and Dosing Strategies
Optimal tracer dosing requires balancing analytical requirements with physiological relevance. For ^13^C-ALA studies, typical doses range from 0.5-5 mg/kg body weight, depending on analytical sensitivity and study duration [41]. Higher doses may be necessary for studies using natural abundance IRMS approaches or when measuring incorporation into pools with very slow turnover rates.
The timing and frequency of sample collection must align with the kinetic characteristics of the metabolic processes under investigation. For rapid kinetic assessment of plasma appearance and disappearance, frequent early sampling (e.g., every 10-30 minutes for the first 4-6 hours) is essential [41]. For longer-term incorporation into tissue pools, less frequent sampling over days or weeks is appropriate, particularly when using D~2~O labeling approaches that provide integrated measures of synthesis over time [42].
Table 2: Comparison of Tracer Administration Methods for ALA Studies
| Parameter | Intravenous Infusion | Oral Administration | Dâ‚‚O Labeling |
|---|---|---|---|
| Study Duration | Hours (typically 2-12) [42] | Hours to days | Days to weeks [42] |
| Metabolic Information | Plasma kinetics, conversion rates, oxidation | Absorption, first-pass metabolism, portal delivery | Long-term incorporation, tissue turnover, total body synthesis |
| Analytical Complexity | High (frequent sampling, steady-state requirements) | Moderate (timed sampling after dose) | Low (minimal sampling after equilibration) |
| Physiological Relevance | Controlled delivery but non-physiological route | Mimics dietary intake | Endogenous synthesis tracking |
| Cost Considerations | High (clinical facility, infusion supplies) | Moderate to high (tracer synthesis) | Low (Dâ‚‚O is relatively inexpensive) |
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Essential Research Reagents for ALA Tracer Studies
| Reagent/Material | Specification Requirements | Application in ALA Research |
|---|---|---|
| Stable Isotope-Labeled ALA | Chemical purity >98%, isotopic purity >99%, position-specific or uniform labeling | Metabolic tracer for kinetics, conversion studies, and metabolic fate mapping |
| Deuterium Oxide (Dâ‚‚O) | 70% or 99% isotopic purity, sterile filtered | Long-term turnover studies, tissue incorporation measurements [42] |
| Lipid Extraction Solvents | HPLC-grade chloroform, methanol, water with antioxidant preservation | Quantitative extraction of lipids from biological samples with minimal oxidation |
| Solid-Phase Extraction Cartridges | Aminopropyl, silica, or reversed-phase chemistries | Fractionation of complex lipid mixtures into classes (phospholipids, triglycerides, etc.) |
| Derivatization Reagents | BSTFA, methanolic HCl, or other methylating reagents | Preparation of volatile derivatives for GC-MS analysis [41] |
| Internal Standards | ^13^C-labeled internal standards for each lipid class of interest | Quantification correction for extraction and processing variability |
| Sample Collection Materials | Vacutainers with antioxidant cocktails (e.g., BHT, EDTA), breath collection bags | Preservation of sample integrity during collection and storage |
| Chromatography Columns | GC capillary columns (high-polarity for FAME), LC C18 or specialized lipid columns | Separation of ALA and metabolites from biological matrices |
| Epigalantamine | Epigalantamine|CAS 1668-85-5|For Research | Epigalantamine, a Galantamine diastereomer for neuroscience research. For Research Use Only. Not for human or veterinary use. |
| Norcyclizine | Norcyclizine, CAS:841-77-0, MF:C17H20N2, MW:252.35 g/mol | Chemical Reagent |
Applications in ALA Health Benefits Research
Cardiovascular Health Applications
Stable isotope tracers have been instrumental in elucidating the mechanisms underlying ALA's cardioprotective effects, which include a 5% reduction in cardiovascular mortality risk per 1 g/day increase in ALA intake [43]. Tracer methodologies have demonstrated that ALA incorporation into cardiac membrane phospholipids influences membrane fluidity, receptor function, and signal transduction pathways [43]. Additionally, isotope studies have revealed ALA's role as a precursor for cardioprotective eicosanoids derived from EPA, providing a metabolic explanation for the observed inverse relationship between ALA intake and coronary heart disease risk (pooled RR 0.89 for highest vs. lowest intake) [43].
The anti-inflammatory properties of ALA and its metabolites have been quantified using tracer approaches, demonstrating dose-dependent incorporation into inflammatory cell membranes and subsequent modification of eicosanoid production profiles [43]. These studies provide a metabolic basis for understanding how ALA intake influences inflammatory pathways implicated in atherosclerosis development and progression.
Dose-Response and Optimal Intake Studies
Stable isotope methodologies enable precise determination of ALA kinetics across a range of intakes, providing critical data for establishing optimal intake recommendations. Dose-response studies using ^13^C-ALA have demonstrated that ALA conversion to EPA becomes less efficient at higher intake levels, suggesting potential saturation of desaturation/elongation enzymes [43]. This information is crucial for developing targeted recommendations for different population subgroups based on their metabolic characteristics and health status.
Competition studies using stable isotopes have illuminated the metabolic interactions between ALA and linoleic acid (LA), demonstrating that high LA intakes can inhibit ALA conversion to EPA and DHA by competing for desaturase enzymes [43]. These findings have important implications for dietary recommendations that consider both absolute intakes and relative ratios of these essential fatty acids.
Advanced Applications and Future Directions
Compound-Specific Isotope Analysis
Advanced IRMS techniques now enable compound-specific isotope analysis of individual lipid species, providing unprecedented resolution in metabolic tracking [45]. This approach allows researchers to trace ALA incorporation into specific lipid classes and molecular species within complex biological samples, revealing previously inaccessible aspects of lipid metabolic routing and compartmentalization. Such detailed metabolic mapping is particularly valuable for understanding the relationship between ALA metabolism and cellular function in different tissues.
Multi-Isotope Tracer Approaches
The simultaneous use of multiple tracers with different isotopic labels or labeling patterns represents a powerful approach for comprehensive metabolic mapping [40]. For ALA research, this might include using ^13^C-ALA in combination with ^2^H-LA to simultaneously track both metabolic fates and their potential competition. These multi-tracer designs can provide more complete metabolic information while reducing subject burden and interindividual variability.
Integration with Omics Technologies
The future of ALA metabolism research lies in integrating stable isotope tracer methodologies with genomics, transcriptomics, and proteomics approaches [46]. This integrated strategy enables researchers to connect metabolic kinetics with their molecular determinants, such as relating ALA conversion efficiency to FADS genotype or desaturase enzyme expression levels [43]. Such comprehensive approaches will ultimately support personalized nutrition recommendations based on individual metabolic characteristics.
Stable isotope tracer methodologies provide an indispensable toolkit for advancing our understanding of ALA metabolism and its relationship to human health. The techniques outlined in this guide enable precise, dynamic assessment of ALA kinetics, metabolic fate, and biological effects, supporting evidence-based recommendations for optimal ALA intake and targeted therapeutic applications.
Alpha-linolenic acid (ALA), an essential omega-3 fatty acid, undergoes complex metabolic partitioning upon ingestion, primarily between mitochondrial β-oxidation for energy production and enzymatic elongation/desaturation to longer-chain polyunsaturated fatty acids (LCPUFAs) like eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Understanding and quantifying this partitioning is critical for nutritional science and therapeutic development, as the resulting metabolites confer distinct health benefits. The balance between these pathways influences the ultimate biological value of dietary ALA, affecting its role in cardiovascular protection, neuroprotection, and anti-inflammatory processes [47] [48] [49]. This guide provides a technical framework for researchers to quantitatively assess these metabolic fates, focusing on experimental methodologies, data interpretation, and the molecular regulation of ALA metabolism.
Core Metabolic Pathways of ALA
The metabolic journey of ALA in humans primarily involves three competitive pathways: β-oxidation, elongation/desaturation, and incorporation into tissue lipids. The elongation/desaturation pathway occurs in the endoplasmic reticulum and involves a series of enzymatic reactions: delta-6 desaturase (D6D) acts on ALA to produce stearidonic acid (18:4n-3), which is then elongated to eicosatosatetraenoic acid (20:4n-3), and finally delta-5 desaturase (D5D) converts it to EPA (20:5n-3). Further elongation and desaturation produce DHA (22:6n-3) [31] [50]. Concurrently, ALA can enter the mitochondria and undergo β-oxidation, being broken down into acetyl-CoA units for energy production or other synthetic processes. These pathways are competitive, as the enzymes involved, particularly D5D and D6D, are shared with the omega-6 fatty acid family, creating a metabolic tug-of-war influenced by dietary intake and genetic factors [31].
The following diagram illustrates the core metabolic partitioning of ALA and its regulatory elements:
Figure 1: ALA Metabolic Partitioning and Regulation. This map illustrates the competitive pathways of β-oxidation, elongation/desaturation, and storage, along with key regulatory factors including dietary ratios and transcriptional regulators.
Quantitative Analysis of Metabolic Partitioning
Quantifying the distribution of ALA between β-oxidation and conversion pathways reveals the significant inefficiency of long-chain PUFA synthesis in humans. The following table summarizes key quantitative findings from experimental studies:
Table 1: Quantitative Partitioning of ALA Metabolism
| Experimental Model | β-Oxidation | Conversion to EPA | Conversion to DHA | Key Influencing Factor | Citation |
|---|---|---|---|---|---|
| Human Hepatoma Cells (HepG2) | Not Quantified | Up to 17% of recovered ALA | 0.7% of recovered ALA | LA/ALA ratio of 1:1 | [31] |
| Human Metabolism | Major fate | Typically < 5-10% | Typically < 0.1-4% | Sex, genetic polymorphisms | [50] |
| General Human Metabolism | Significant portion recycled for de novo lipogenesis | Limited capacity | Very limited capacity | High LA intake reduces conversion | [50] |
The data demonstrates that β-oxidation constitutes the major metabolic fate of dietary ALA, while conversion to DHA is particularly limited. The efficiency of conversion is highly dependent on external factors, most notably the ratio of competing omega-6 linoleic acid (LA) to ALA in the diet. Research using human hepatoma cells incubated with varying ratios of labeled LA and ALA demonstrated that the conversion efficiency peaks at a LA/ALA ratio of 1:1, with 17% of recovered ALA converted to EPA and 0.7% to DHA. This optimal ratio is far from the 10:1 to 25:1 ratio typical of Western diets, which substantially suppresses ALA conversion [31]. This competitive inhibition occurs because LA and ALA vie for the same enzymatic machinery (D5D and D6D).
Beyond dietary composition, intrinsic biological factors significantly influence partitioning. A comprehensive metabolic review noted that sex differences exist in ALA metabolism, with women often demonstrating higher conversion rates to DHA, potentially linked to estrogen effects [50]. Furthermore, genetic polymorphisms in the desaturase enzymes (FADS1 and FADS2 genes) lead to population-wide variations in conversion capacity.
Experimental Protocols for Quantification
In Vitro Cell Culture Model with Stable Isotopes
Objective: To quantitatively track the conversion of ALA to EPA and DHA in a controlled cellular environment, assessing the impact of different n6/n3 fatty acid ratios.
Detailed Methodology:
Cell Culture Setup:
- Utilize human hepatoma HepG2 cells as a model for human liver metabolism.
- Culture cells in T-25 flasks at a density of 5 x 10^6 cells in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 20% fetal calf serum (FCS), 2% glutamine, and 2% penicillin/streptomycin at 37°C in a 5% CO2 atmosphere [31].
Treatment Preparation:
- Prepare mixtures of [13C]-labeled linoleic acid (LA) and [13C]-labeled alpha-linolenic acid (ALA) methyl esters suspended in sterile bovine serum albumin (BSA, Fraction V).
- Define experimental groups with a constant total fatty acid concentration of 100 μM but varying LA/ALA ratios (e.g., 9:1, 4:1, 1:1, ALA only) to model different dietary inputs [31].
Cell Exposure and Harvesting:
- Replace the growth medium with 8 ml of serum-free DMEM 24 hours before treatment.
- Add the prepared fatty acid-BSA complexes to the cells and incubate for 24 hours. Perform each condition in quadruplicate for statistical power.
- After incubation, harvest cells using accutase, collect the medium separately, and centrifuge the cell suspension at 500 x g. Wash the cell pellet with saline and store at -75°C [31].
Lipid Extraction and Analysis:
- Extract lipids from cell pellets using a modified Folch extraction (chloroform:methanol, 2:1 v/v) with the addition of internal standards (e.g., heptadecanoic acid and tetracosanoic acid) for quantification [31].
- Analyze fatty acid methyl esters (FAMEs) using Gas Chromatography-Mass Spectrometry (GC-MS). The [13C] label allows for precise tracking of the metabolic fate of the administered ALA distinct from endogenous pools.
Data Calculation:
- Quantify the percentage of recovered [13C]ALA that was converted to [13C]EPA and [13C]DHA using the peak areas from GC-MS analysis, normalized to the internal standards.
In Vivo Metabolic Tracer Studies
Objective: To measure the whole-body partitioning of ALA in human subjects, including β-oxidation and conversion rates.
Detailed Methodology:
Study Design and Tracer Administration:
- Conduct a randomized, controlled dietary intervention where subjects follow a standardized diet for a wash-in period (e.g., 3 days) [51].
- Administer a bolus of [U-13C]ALA orally to participants. The dose is typically calculated based on body weight.
Sample Collection:
- Collect blood samples at multiple time points over a defined period (e.g., 24-48 hours) to track the appearance and disappearance of the tracer and its metabolites in plasma phospholipids, cholesteryl esters, and triglycerides.
- In specialized settings (e.g., respiration chambers), collect breath samples to measure 13CO2, which is a direct product of the β-oxidation of the labeled ALA [51].
Sample Processing and Analysis:
- Extract lipids from plasma samples and separate lipid classes via thin-layer chromatography (TLC) or solid-phase extraction.
- Prepare FAMEs from the isolated lipid fractions and analyze via GC-MS or LC-MS to quantify the enrichment of 13C in ALA, EPA, DHA, and other metabolites.
- Analyze breath 13CO2 enrichment using Isotope Ratio Mass Spectrometry (IRMS).
Compartmental Modeling:
- Use the tracer enrichment data in plasma and breath as input for compartmental modeling software. This mathematical approach estimates kinetic parameters such as the fractional conversion rate (FCR) of ALA to EPA/DHA and the absolute rate of ALA β-oxidation [52].
The following workflow diagram visualizes the key stages of these experimental approaches:
Figure 2: Experimental Workflows for Quantifying ALA Partitioning. The diagram outlines parallel in vivo and in vitro approaches using stable isotopes and mass spectrometry to determine β-oxidation and conversion rates.
Molecular Regulation of Partitioning
The metabolic partitioning of ALA is not a passive process but is actively regulated by cellular signaling pathways and transcription factors that respond to the fatty acid milieu.
Transcriptional Control: Key transcription factors include Peroxisome Proliferator-Activated Receptor Alpha (PPARα), which promotes genes involved in fatty acid β-oxidation, and Sterol Regulatory Element-Binding Protein 1c (SREBP-1c), which is involved in fatty acid synthesis and has been shown to regulate expression of D5D and D6D [31]. The expression of these transcription factors themselves is influenced by fatty acids. Research in HepG2 cells has demonstrated that the ratio of n6/n3 fatty acids in the cellular environment differentially regulates the transcript levels of PPARα, SREBP-1c, D5D, and D6D [31].
Signaling Pathways: The Mitogen-Activated Protein Kinase (MAPK) pathways, including MEK and MEKK, are involved in this regulatory network. These kinases can phosphorylate and modify the activity of both PPARs and SREBPs, creating a complex web of post-translational control that fine-tunes the metabolic response to ALA and other fatty acids [31] [53]. Essential fatty acids like LA and ALA have been shown to induce MAPK phosphorylation, thereby linking initial nutrient sensing to long-term adaptive changes in gene expression [31].
The following map summarizes the interplay of these regulatory elements:
Figure 3: Signaling Pathways Regulating ALA Partitioning. This map shows how dietary fatty acid ratios are sensed by the cell, triggering MAPK-mediated signaling that influences key transcription factors, ultimately shifting the balance between β-oxidation and conversion pathways.
The Scientist's Toolkit: Essential Research Reagents and Platforms
Successful investigation into ALA metabolism requires a suite of specialized reagents, tools, and bioinformatics platforms. The following table details key resources for designing experiments in this field.
Table 2: Essential Research Reagents and Resources
| Tool / Resource | Specific Example | Function in ALA Metabolism Research |
|---|---|---|
| Stable Isotope Tracer | [13C]-Uniformly Labeled ALA | Enables precise tracking of ALA carbon fate through β-oxidation (as 13CO2) and conversion pathways (as labeled EPA/DHA) in both in vivo and in vitro systems. |
| Cell Culture Model | Human Hepatoma HepG2 Cell Line | Provides a controlled human-relevant system to study hepatic conversion of ALA to EPA/DHA and the impact of genetic or pharmacological perturbations. |
| Analytical Instrumentation | GC-MS / LC-MS | The core platform for separating, detecting, and quantifying fatty acids and their labeled counterparts in complex biological samples with high sensitivity and specificity. |
| Bioinformatics Database | KEGG PATHWAY (e.g., map01050) | Provides reference maps for glycerolphospholipid metabolism and unsaturated fatty acid biosynthesis, allowing researchers to contextualize their findings within established pathways [53]. |
| Metabolomics Analysis Suite | MetaboAnalyst | A web-based platform for comprehensive statistical analysis of metabolomics data, including pathway analysis and visualization of fatty acid changes [54]. |
| Pathway & Enzyme Database | MetaCyc | A curated database of experimentally elucidated metabolic pathways and enzymes, useful for verifying and exploring the enzymes involved in ALA elongation and desaturation [55]. |
| Bis(2-chloroethyl)amine hydrochloride | Bis(2-chloroethyl)amine hydrochloride, CAS:821-48-7, MF:C4H9Cl2N.ClH, MW:178.48 g/mol | Chemical Reagent |
| Doxorubicin | Doxorubicin, CAS:25316-40-9, MF:C27H29NO11, MW:543.5 g/mol | Chemical Reagent |
The quantitative partitioning of ALA between β-oxidation and conversion to long-chain PUFAs is a central determinant of its efficacy in promoting health. The data unequivocally shows that while β-oxidation is the dominant pathway, the conversion to EPA and DHA, though inefficient, is critically dependent on dietary context—specifically, a balanced n6/n3 fatty acid intake. The experimental frameworks outlined, employing stable isotopes and advanced mass spectrometry, provide robust methodologies for quantifying these metabolic fluxes. A deep understanding of this partitioning, coupled with insights into its molecular regulation, is indispensable for developing targeted nutritional strategies and therapeutic interventions aimed at optimizing the health benefits of plant-derived omega-3 fatty acids. Future research integrating multi-omics approaches will further refine our understanding of individual variability in ALA metabolism.
Analytical Techniques for Measuring ALA and Metabolites in Tissues
Alpha-linolenic acid (ALA) is an essential C-18 n-3 polyunsaturated fatty acid (PUFA) that serves as a crucial precursor for longer n-3 PUFAs like eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [56]. As research continues to uncover the health benefits of ALA in cardiovascular disease prevention, cognitive health support, and inflammation mitigation [56] [57] [58], the demand for precise analytical techniques to measure ALA and its metabolites in tissues has grown significantly. The complexity of ALA metabolism, including its conversion to bioactive oxylipins such as 9-HOTrE and 13-HOTrE through lipoxygenase (LOX), cyclooxygenase (COX), and cytochrome P450 (CYP450) pathways, presents unique challenges for researchers [56]. This technical guide provides an in-depth examination of current methodologies for analyzing ALA and its metabolic derivatives in tissue samples, offering detailed protocols and analytical frameworks to support advancement in nutritional science, pharmacology, and clinical research.
Major Analytical Platforms and Techniques
Mass Spectrometry Imaging for Spatial Metabolomics
Matrix-assisted laser desorption ionization mass spectrometry imaging (MALDI-MSI) has emerged as a powerful label-free molecular imaging technique that enables direct and simultaneous mapping of hundreds of different metabolites in thin tissue sections [59]. This approach preserves spatial information that is lost in conventional extraction-based metabolomics, allowing researchers to localize ALA and its metabolites within specific tissue microenvironments.
The metaFISH protocol represents a significant advancement in this field, combining MALDI-MSI with fluorescence in situ hybridization (FISH) to correlate metabolic distributions with microbial localization in host-microbe interactions [59]. This correlative approach is particularly valuable for investigating how gut microbiota influences ALA metabolism and bioavailability, as demonstrated in studies linking Phascolarctobacterium abundance with ALA levels [60]. Modern MALDI-MSI systems achieve pixel sizes of 5-10 μm, sufficient to differentiate colonized and uncolonized eukaryotic cells, with experimental transmission mode devices achieving subcellular resolution below 1 μm [59].
Protocol: MetaFISH for Spatial Localization of ALA Metabolites Sample Preparation:
- Embed fresh tissue specimens in optimal cutting temperature (OCT) compound and snap-freeze in liquid nitrogen-cooled isopentane
- Section tissues at 5-10 μm thickness using a cryostat
- Thaw-mount sections onto pre-chilled ITO-coated glass slides or standard glass slides for MALDI-MSI
MALDI-MSI Data Acquisition:
- Apply matrix (e.g., DHB for negative ion mode) using automated sprayer system (20 mg/mL in 70% methanol)
- Acquire data using MALDI mass spectrometer with resolution set to 5-10 μm pixel size
- Set mass range to m/z 50-1000 for comprehensive metabolite detection
- For ALA (m/z 278.2) and oxylipin detection, use negative ion mode with laser intensity optimized for lipid detection
Post-MSI Fixation and FISH:
- Fix tissue sections in 4% paraformaldehyde for 1 hour after MSI acquisition
- Perform on-tissue hybridization with specific 16S rRNA FISH probes for bacterial identification
- Incubate with fluorescently labeled probes overnight at 46°C in hybridization buffer
Data Integration:
- Correlate fluorescence microscopy images with MALDI-MSI data using registration algorithms
- Overlay metabolic heat maps with microbial localization data
- Identify metabolite-bacteria associations through spatial correlation analysis [59]
Liquid Chromatography-Mass Spectrometry Approaches
Liquid chromatography coupled to mass spectrometry (LC-MS) remains the workhorse technology for targeted and untargeted analysis of ALA and its metabolites from tissue extracts. Two primary approaches dominate this field: untargeted metabolomics for discovery and targeted methods for quantitative analysis.
Untargeted Metabolomics Protocol: Sample Extraction:
- Homogenize 20-50 mg tissue in 500 μL methanol:acetonitrile:water (2:2:1, v/v) with internal standards (e.g., L-2-chlorophenylalanine)
- Utilize bead beating at 45 Hz for 4 minutes followed by ice-water bath sonication for 5 minutes (repeat 3x)
- Centrifuge at 12,000 rpm for 15 minutes at 4°C
- Collect supernatant and dry in vacuum concentrator
- Reconstitute in 150 μL acetonitrile:water (1:1, v/v) for LC-MS analysis [60]
LC-MS Analysis:
- Employ UPLC system with HSS T3 column (1.8 μm, 2.1×100 mm)
- Use mobile phase A: 0.1% formic acid in water; mobile phase B: 0.1% formic acid in acetonitrile
- Apply gradient: 0-0.25 min (98% A), 0.25-10 min (2% A), 10-13 min (2% A), 13-13.1 min (98% A), 13.1-15 min (98% A)
- Operate mass spectrometer in both positive and negative ionization modes with data-independent acquisition (MSe mode)
- Maintain flow rate at 400 μL/min with injection volume of 1-3 μL [60]
Data Processing:
- Use Progenesis QI or similar software for peak picking, alignment, and normalization
- Perform multivariate statistical analysis (PCA, OPLS-DA) to identify significant metabolites
- Annotate metabolites using accurate mass, MS/MS fragmentation, and database searching (HMDB, LipidMaps)
Targeted Metabolomics Protocol for ALA Oxylipins: Sample Preparation:
- Add deuterated internal standards (e.g., d8-ARA, d4-PGE2) prior to extraction
- Perform solid-phase extraction using C18 or mixed-mode cartridges for oxylipin enrichment
- Derivatize with AMPP or other reagents to enhance sensitivity if required
LC-MRM Analysis:
- Utilize hydrophilic interaction liquid chromatography (HILIC) for polar metabolites or reversed-phase for oxylipins
- Configure triple quadrupole mass spectrometer for multiple reaction monitoring (MRM)
- Monitor specific transitions for ALA metabolites:
- ALA: m/z 277.2→233.2 (collision energy -18 eV)
- 13-HOTrE: m/z 293.2→195.1 (collision energy -20 eV)
- 9-HOTrE: m/z 293.2→229.1 (collision energy -18 eV)
- Employ polarity switching to detect both positive and negative ions in single run [61]
Quantification:
- Generate calibration curves using authentic standards (0.1-500 ng/mL)
- Apply internal standard correction for recovery and matrix effects
- Validate method for precision (RSD <15%), accuracy (85-115%), and sensitivity (LLOQ ~0.1 ng/mL)
Advanced Correlative Imaging Approaches
The integration of MALDI-MSI with other spatial technologies provides unprecedented insights into ALA metabolism within tissue contexts. Microcomputed tomography (μCT) can be combined with MSI and FISH to create three-dimensional atlases of metabolic interactions, particularly valuable for understanding ALA distribution in relation to anatomical structures [59]. This approach is especially powerful for investigating how ALA and its metabolites, such as the anti-inflammatory 13-(S)-HPOTrE and 13-(S)-HOTrE, distribute within inflamed tissues and interact with immune cells [58].
Technical Comparison of Analytical Approaches
Table 1: Comparison of Major Analytical Techniques for ALA and Metabolite Analysis
| Technique | Spatial Resolution | Metabolite Coverage | Quantitation Capability | Throughput | Best Applications |
|---|---|---|---|---|---|
| MALDI-MSI | 5-10 μm (commercial), <1 μm (experimental) | 100-500 metabolites | Semi-quantitative with internal standards | Medium | Spatial distribution, host-microbe interactions, metabolite localization [59] |
| LC-MS Untargeted | None (tissue homogenate) | 500-1000+ metabolites | Relative quantitation | High | Discovery studies, pathway analysis, biomarker identification [60] |
| LC-MS Targeted (MRM) | None (tissue homogenate) | 10-200 metabolites | Absolute quantitation | High | Validation studies, oxylipin profiling, clinical applications [61] |
| metaFISH | Single-cell (1 μm) | 100-300 metabolites + microbial identification | Semi-quantitative | Low | Correlating metabolites with specific microbes, functional microbiomics [59] |
Table 2: Key ALA Metabolites and Their Mass Spectrometric Detection Parameters
| Metabolite | Formula | m/z [M-H]- | Retention Time (RP-LC) | Characteristic Fragments | Biological Significance |
|---|---|---|---|---|---|
| ALA | C18H30O2 | 277.2 | 12.5 min | 233.2, 205.1, 149.0 | Essential fatty acid precursor [56] |
| 13-HOTrE | C18H30O3 | 293.2 | 9.8 min | 195.1, 177.1, 135.1 | Anti-inflammatory, inactivates NLRP3 inflammasome [58] |
| 9-HOTrE | C18H30O3 | 293.2 | 10.2 min | 229.1, 171.1, 125.1 | Immunomodulating effects [56] |
| 13-HPOTrE | C18H30O4 | 309.2 | 8.5 min | 211.1, 193.1, 175.1 | Potent anti-inflammatory precursor [58] |
| EpODE | C18H30O3 | 293.2 | 11.1 min | 275.2, 257.2, 201.1 | CYP450 pathway metabolite |
Research Reagent Solutions
Table 3: Essential Research Reagents for ALA Metabolite Analysis
| Reagent/Category | Specific Examples | Function/Application | Technical Notes |
|---|---|---|---|
| Internal Standards | d5-ALA, d4-13-HOTrE, d8-ARA | Quantitation standardization, recovery correction | Use stable isotope-labeled versions for minimal matrix effects |
| MS Matrices | DHB (2,5-dihydroxybenzoic acid), CHCA (α-cyano-4-hydroxycinnamic acid) | Facilitate laser desorption/ionization in MALDI-MSI | DHB preferred for lipids in negative mode; optimize concentration (20 mg/mL) [59] |
| Chromatography Columns | HSS T3 (C18), BEH Amide (HILIC) | Metabolite separation prior to MS detection | HILIC for polar oxylipins; C18 for ALA and hydroxy metabolites [60] [61] |
| FISH Probes | 16S rRNA-targeted probes (e.g., EUB338, NON338) | Microbial identification and localization in tissues | Design species-specific probes based on 16S sequences; optimize hybridization temperature [59] |
| Extraction Solvents | Methanol:acetonitrile:water (2:2:1) | Metabolite extraction from tissues | Pre-chill to -20°C; include antioxidant (BHT) for oxylipin preservation [60] |
| Authentic Standards | ALA, 13(S)-HOTrE, 9(S)-HOTrE, DiHOTrE | Metabolite identification and quantitation | Source from specialized manufacturers; prepare fresh stock solutions in ethanol |
Analytical Workflows and Signaling Pathways
Integrated Workflow for Comprehensive ALA Metabolite Analysis
The following diagram illustrates the comprehensive workflow for analyzing ALA and its metabolites in tissues, integrating both spatial and extraction-based approaches:
ALA Metabolic Pathways and Signaling Mechanisms
The diagram below illustrates the metabolic fate of ALA and the signaling pathways modulated by its oxylipin metabolites:
Methodological Considerations and Challenges
Pre-analytical Variables
The analysis of ALA and its metabolites requires careful attention to pre-analytical factors that can significantly impact results. Tissue collection should be performed rapidly with immediate snap-freezing in liquid nitrogen to prevent ongoing enzymatic activity that may alter metabolite profiles [59]. The addition of antioxidants such as butylated hydroxytoluene (BHT) to extraction solvents is essential to prevent oxidation of PUFAs during processing [60]. For spatial analysis, optimal cutting temperature (OCT) compound should be used judiciously as it can interfere with MS analysis if not completely removed from tissue sections.
Analytical Optimization Strategies
Signal suppression in mass spectrometry can be mitigated through efficient chromatographic separation and clean-up procedures. For LC-MS analysis, matrix effects should be evaluated by comparing standards in neat solvent versus tissue matrix, with stable isotope-labeled internal standards providing correction for ionization efficiency variations [61]. In MALDI-MSI, matrix application uniformity is critical for quantitative comparisons across tissue regions, requiring automated sprayers with controlled temperature and flow rate parameters [59].
Data Integration and Interpretation
The complexity of ALA metabolic networks necessitates sophisticated data analysis approaches. Integration of multiple datasets (spatial, quantitative, microbial) requires specialized bioinformatics tools and statistical methods. Pathway analysis software such as MetaboAnalyst can help contextualize measured metabolites within biological processes, while spatial correlation algorithms enable identification of metabolite-microbe relationships in tissue environments [59] [62].
The analytical techniques for measuring ALA and its metabolites in tissues have evolved significantly, enabling researchers to address increasingly complex biological questions about ALA metabolism and its health benefits. The integration of spatial techniques like MALDI-MSI with quantitative LC-MS methods and microbial localization approaches provides a comprehensive toolkit for unraveling the complex interplay between ALA, its bioactive metabolites, and physiological outcomes. As these methodologies continue to advance, particularly through improvements in spatial resolution, sensitivity, and computational integration, researchers will be better positioned to develop targeted nutritional and therapeutic strategies leveraging the health-promoting properties of ALA and its metabolic derivatives. The continued refinement of these analytical platforms will undoubtedly yield new insights into ALA metabolism and expand its applications in preventive medicine and therapeutic interventions.
ALA's Cardioprotective Mechanisms and Clinical Evidence
Alpha-linolenic acid (ALA), an essential omega-3 polyunsaturated fatty acid, has emerged as a significant nutrient in cardiovascular disease prevention and management. As the primary plant-derived omega-3 fatty acid, ALA serves as a crucial precursor to longer-chain metabolites including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), though it also exhibits independent biological activity. Cardiovascular diseases (CVDs) represent the leading cause of global mortality, responsible for approximately 1.7 million deaths annually, creating an urgent need for effective preventive and therapeutic nutritional strategies [63]. The cardioprotective potential of ALA has been demonstrated through multiple research avenues, including epidemiological studies, randomized controlled trials, and investigations into its fundamental molecular mechanisms. This whitepaper synthesizes current scientific evidence regarding ALA's cardiovascular benefits, focusing on its lipid-modifying, anti-arrhythmic, anti-inflammatory, and antihypertensive properties, while also examining the practical application of this knowledge in clinical and research settings. Understanding ALA's complete cardioprotective profile is particularly valuable given increasing concerns about marine source sustainability and for populations with limited fish consumption, positioning ALA-rich plant sources as viable alternatives for obtaining cardiovascular benefits associated with omega-3 fatty acids [49].
Molecular Mechanisms of Cardioprotection
Lipid Metabolism Modulation
ALA exerts significant influence on circulating lipid profiles, particularly affecting low-density lipoprotein cholesterol (LDL-C) and triglyceride concentrations. The lipid-lowering effects represent one of the most thoroughly documented cardioprotective mechanisms of ALA. Recent large-scale prospective cohort research involving 117,871 individuals from the UK Biobank has demonstrated that higher circulating levels of polyunsaturated fatty acids, including ALA, are inversely associated with cardiovascular mortality [64]. This extensive biomarker study revealed that plasma PUFAs were associated with lower total and cardiovascular mortality, with the protective associations mainly mediated through apolipoprotein A (ApoA) and C-reactive protein (CRP) levels [64]. The cholesterol-lowering effects of ALA may be attributed to multiple factors, including enhanced LDL receptor activity, reduced hepatic very-low-density lipoprotein (VLDL) production, and increased clearance of circulating lipoproteins.
Anti-arrhythmic Effects
The fundamental anti-arrhythmic properties of ALA represent another crucial cardioprotective mechanism, particularly relevant to the prevention of sudden cardiac death. Experimental studies using animal models and in vitro investigations on cultured myocytes have consistently demonstrated that ALA effectively prevents ventricular fibrillation, which is the primary mechanism underlying cardiac death [65]. Interestingly, comparative research has suggested that among omega-3 fatty acids, ALA may be more efficient than EPA and DHA in preventing ventricular fibrillation [65]. This direct anti-arrhythmic action at the cardiac myocyte level complements ALA's other cardiovascular benefits and may explain the significant reduction in cardiac mortality observed in intervention studies where diets were enriched with ALA, as opposed to those merely enriched with linoleic acid [65].
Anti-inflammatory and Antithrombotic Actions
ALA contributes to the reduction of chronic inflammation and thrombosis through multiple interconnected pathways. The anti-inflammatory effects are partially mediated through ALA's role as a precursor to EPA, which gives rise to eicosanoids with lower inflammatory potential compared to those derived from arachidonic acid [63]. ALA has been demonstrated to be the primary fatty acid responsible for reducing platelet aggregation, a critical step in thrombosis that contributes to non-fatal myocardial infarction and stroke [65]. Additionally, ALA intake has been inversely associated with serum C-reactive protein levels, a key marker of systemic inflammation [64]. The balanced intake of ALA and linoleic acid is critical for achieving optimal anti-inflammatory effects, with research suggesting that maintaining an omega-6/omega-3 ratio between 5:1 and 10:1 promotes cardiovascular health [63].
Blood Pressure Regulation
Hypertension represents a major modifiable risk factor for cardiovascular diseases, and ALA demonstrates beneficial effects on blood pressure regulation. Although much research has focused on alpha-lipoic acid (which is distinct from alpha-linolenic acid), emerging evidence suggests that ALA contributes to cardiovascular health through multiple mechanisms that indirectly influence blood pressure, including improved endothelial function, enhanced nitric oxide bioavailability, and reduced systemic inflammation [63] [65]. The consumption of ALA-rich foods has been associated with improved vascular reactivity and arterial compliance, potentially contributing to better blood pressure control and reduced hypertension risk.
Clinical Evidence and Epidemiological Data
Intervention Trials and Clinical Outcomes
Table 1: Clinical Outcomes from ALA Intervention Studies
| Study Reference | Population | Intervention | Key Findings | Risk Reduction |
|---|---|---|---|---|
| Jiao et al. [64] | 117,871 adults | Circulating ALA levels | Inverse association with CVD mortality | Significant trend across quartiles |
| de Lorgeril et al. [65] | Coronary patients | ALA-enriched diet | Reduction in cardiac death | Particularly sudden death |
| 2009 EFSA Statement [63] | General population | ALA consumption | Reduced CVD risk | Based on anti-hypertensive, anti-atherosclerotic effects |
Clinical evidence from intervention trials provides compelling support for ALA's cardioprotective effects. The landmark study by de Lorgeril et al. demonstrated that dietary enrichment with ALA, unlike linoleic acid supplementation alone, significantly reduced cardiac mortality, particularly sudden death [65]. This finding highlights the specific anti-arrhythmic properties of ALA that extend beyond general cholesterol-lowering effects. More recent evidence from large prospective cohorts has reinforced these findings, showing that circulating ALA levels are inversely associated with cardiovascular mortality in a dose-response manner [64]. The European Food Safety Authority (EFSA) has acknowledged these cardiovascular benefits, stating that dietary ALA may contribute to reducing CVD risk through multiple mechanisms including anti-hypertensive, anti-atherosclerotic, and direct cardioprotective effects [63].
Biomarker Studies and Circulating ALA
Table 2: Association of Circulating Fatty Acids with Mortality Outcomes
| Fatty Acid Category | Total Mortality | CVD Mortality | Cancer Mortality | Primary Food Sources |
|---|---|---|---|---|
| Total n-3 PUFAs | Inverse association | Inverse association | Inverse association | Fish, flaxseed, walnuts |
| ALA | Inverse association | Inverse association | Not significant | Flaxseed, walnuts, canola oil |
| Linoleic Acid (LA) | Inverse association | Inverse association | Not significant | Vegetable oils, nuts |
| Total SFAs | Positive association | Not significant | Not significant | Animal fats, tropical oils |
Recent advances in metabolic profiling have enabled more precise characterization of the relationship between circulating ALA levels and cardiovascular outcomes. The large-scale prospective cohort study by Jiao et al. utilizing UK Biobank data demonstrated that plasma phospholipid ALA levels were inversely associated with total and cardiovascular disease mortality [64]. This biomarker approach provides significant advantages over traditional dietary recall methods, as circulating fatty acid levels objectively reflect integrated exposure from dietary intake, endogenous synthesis, and metabolic processes [64]. The study further revealed that the protective associations of ALA were partially mediated through apolipoprotein A and C-reactive protein, suggesting complex mechanisms involving both lipid metabolism and inflammation pathways [64].
Experimental Models and Methodologies
In Vitro Cardioprotection Assays
Experimental investigations into ALA's cardioprotective mechanisms have employed sophisticated in vitro models to elucidate fundamental cellular processes. Myocyte culture systems have been particularly valuable for studying ALA's anti-arrhythmic properties, with research demonstrating that ALA prevents ventricular fibrillation in cultured cardiac cells [65]. These experimental setups typically involve exposing cardiomyocytes to various fatty acid compositions and assessing electrophysiological parameters, calcium handling, and response to arrhythmogenic stimuli. Additional in vitro methodologies include measuring the impact of ALA on endothelial function through nitric oxide production, assessment of inflammatory markers in endothelial cells, and evaluation of gene expression changes in response to ALA supplementation. These controlled systems allow researchers to isolate specific mechanisms without the confounding factors present in whole-organism studies.
In Vivo Animal Studies
Animal models have been instrumental in establishing ALA's cardioprotective potential and elucidating underlying mechanisms. Studies in rats have demonstrated that ALA effectively prevents ventricular fibrillation, with comparative research suggesting that ALA may be more efficient than EPA and DHA in this regard [65]. Typical experimental protocols involve dietary interventions with varying ALA content, followed by induction of ischemia-reperfusion injury or arrhythmia through coronary artery ligation or other techniques. Outcome measures commonly include infarct size quantification, arrhythmia incidence and severity, cardiac function assessment through echocardiography, and tissue analysis for molecular markers. These animal studies have been crucial for establishing causal relationships and dose-response effects that would be difficult to demonstrate in human trials.
Human Clinical Trial Designs
Randomized controlled trials represent the gold standard for evaluating ALA's cardioprotective effects in human populations. These studies typically employ parallel-group or crossover designs with participants randomly assigned to receive either ALA supplementation or control/placebo. Optimal dosing strategies based on current evidence suggest that ALA intake of approximately 2 grams per day provides cardioprotective benefits [65]. Intervention durations in clinical trials have ranged from several weeks to multiple years, with longer trials necessary for assessing hard cardiovascular endpoints. Methodological considerations include careful assessment of background diet, particularly the balance between omega-6 and omega-3 fatty acids, as the LA/ALA ratio significantly influences physiological effects [63]. Successful trial designs typically include precise assessment of ALA status through circulating biomarkers, standardized cardiovascular outcome measures, and adequate statistical power to detect clinically relevant differences.
Research Reagent Solutions
Table 3: Essential Research Materials for ALA Investigations
| Reagent/Category | Specific Examples | Research Application | Key Function |
|---|---|---|---|
| ALA Sources | Flaxseed oil, hempseed oil, canola oil | Dietary interventions | Provide standardized ALA delivery |
| Control Fats | Olive oil, safflower oil, lard | Control diets | Maintain isolipidic conditions |
| Biomarker Kits | Phospholipid fractionation kits, CRP ELISA | Status assessment | Quantify circulating ALA and inflammation |
| Cell Culture Models | H9c2 cardiomyoblasts, human endothelial cells | In vitro mechanisms | Study cellular responses |
| Animal Models | Rats, mice (wild-type and transgenic) | In vivo cardioprotection | Evaluate integrated physiological effects |
The investigation of ALA's cardioprotective properties requires carefully selected research reagents and model systems. For in vitro studies, H9c2 cardiomyoblast cells serve as a valuable model for investigating molecular mechanisms, while primary human endothelial cells enable research into vascular effects [65] [64]. Animal models, particularly rats, have proven responsive for studying ALA's anti-arrhythmic effects [65]. For human studies, standardized ALA sources including flaxseed oil, hempseed oil, and canola oil provide consistent intervention materials, with appropriate control fats such as olive oil or safflower oil necessary for isolipidic study designs [63]. Advanced lipid profiling technologies, including nuclear magnetic resonance spectroscopy and gas chromatography-mass spectrometry, enable comprehensive characterization of fatty acid profiles and metabolic consequences [66]. Additionally, specific biomarker assessment kits for C-reactive protein, apolipoproteins, and other relevant parameters facilitate the investigation of potential mediating factors in ALA's cardioprotective effects [64].
Metabolic Pathways and Signaling Mechanisms
Diagram 1: ALA Metabolic Pathways and Cardioprotective Mechanisms
The metabolic fate and signaling pathways of ALA underlie its diverse cardioprotective effects. As illustrated in Diagram 1, ALA serves as a precursor for longer-chain omega-3 fatty acids through a series of elongation and desaturation reactions mediated by enzymes including Δ6-desaturase, which competes with linoleic acid for the same enzymatic machinery [63]. The resulting EPA and DHA subsequently incorporate into cell membranes, influencing fluidity, flexibility, and permeability while also serving as precursors for eicosanoid signaling molecules that are less inflammatory than those derived from arachidonic acid [63]. ALA also directly influences gene expression through activation of transcription factors such as PPARs (peroxisome proliferator-activated receptors), leading to modified expression of genes involved in lipid metabolism and inflammation [63]. The anti-arrhythmic effects of ALA operate through both direct modulation of cardiac ion channels and indirect effects through incorporation into membrane phospholipids, thereby stabilizing electrical activity [65]. These multifaceted mechanisms collectively contribute to ALA's ability to reduce cardiovascular risk through lipid-lowering, anti-inflammatory, antithrombotic, and anti-arrhythmic effects.
The substantial body of evidence examining alpha-linolenic acid's cardioprotective properties supports its importance as an essential nutrient with significant potential for cardiovascular risk reduction. The mechanisms underlying these benefits are multifaceted, encompassing lipid-modifying effects, anti-arrhythmic properties, anti-inflammatory actions, and antithrombotic effects. Clinical evidence from intervention trials and large prospective cohort studies demonstrates that ALA consumption, particularly at levels around 2 grams per day with careful attention to the omega-6/omega-3 ratio, is associated with reduced cardiovascular mortality [65] [63]. The translation of these research findings into clinical practice and public health recommendations offers a promising approach to cardiovascular disease prevention, particularly for individuals with limited fish consumption or concerns about marine sustainability. Future research directions should include further elaboration of the molecular mechanisms underlying ALA's cardioprotective effects, optimization of dosing strategies for specific patient populations, and investigation of potential interactions between ALA and genetic polymorphisms affecting fatty acid metabolism. As precision nutrition approaches continue to evolve, ALA is positioned to remain a crucial component of cardiovascular health strategies based on its established efficacy and favorable safety profile.
Anti-inflammatory and Immunomodulatory Properties
In the pursuit of novel therapeutic strategies, the investigation of natural compounds and their influence on fundamental physiological processes has gained significant momentum. Within this context, the anti-inflammatory and immunomodulatory properties of various substances represent a critical area of research, intersecting with the study of alpha-linolenic acid (ALA) metabolism and its extensive health benefits. Inflammation and immune dysregulation underlie a vast spectrum of chronic diseases, from metabolic and cardiovascular disorders to neurodegenerative conditions. Traditional synthetic immunomodulatory medications, while effective, are often accompanied by substantial side effects and high costs, driving the search for safer, multi-target alternatives from natural sources [67]. This whitepaper provides a technical exploration of the mechanisms underlying anti-inflammatory and immunomodulatory effects, with particular attention to how these pathways are influenced by natural compounds and their metabolic interplay. It is structured to serve as a definitive guide for researchers and drug development professionals, integrating detailed mechanistic data, experimental protocols, and visualization of key pathways to support advanced research and therapeutic development.
Molecular Mechanisms of Action
Key Signaling Pathways and Molecular Targets
The anti-inflammatory and immunomodulatory effects of bioactive compounds are primarily mediated through the modulation of critical signaling pathways and transcription factors. The following table summarizes the primary molecular targets and their functional roles in immune regulation.
Table 1: Key Molecular Targets in Anti-inflammatory and Immunomodulatory Processes
| Target Gene/Protein | Full Name | Primary Function in Immunity & Inflammation | Key Modulators |
|---|---|---|---|
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells | Master regulator of pro-inflammatory gene expression; controls cytokines, chemokines, adhesion molecules [68]. | Alpha-lipoic acid (inhibits IKK) [68]; Silymarin constituents [67]. |
| Nrf2 | Nuclear Factor Erythroid 2–Related Factor 2 | Central transcription factor controlling antioxidant response element (ARE)-driven gene expression [69]. | Alpha-lipoic acid (activates via Keap1 modification) [68]. |
| AKT1 | AKT Serine/Threonine Kinase 1 | Regulates cell survival, proliferation, and metabolism; node in PI3K-AKT signaling pathway [67]. | Apigenin, Luteolin, Silibinin (from network pharmacology studies) [67]. |
| PTGS2 | Prostaglandin-Endoperoxide Synthase 2 (COX-2) | Enzyme catalyzing prostaglandin synthesis; key mediator of inflammation and pain [67]. | Apigenin, Luteolin (inhibition via molecular docking) [67]. |
| CASP3 | Caspase 3 | Effector caspase in apoptosis pathway; implicated in inflammatory cell death [67]. | Silibinin (stabilized interactions predicted) [67]. |
| PPARs | Peroxisome Proliferator-Activated Receptors | Nuclear receptors that regulate fatty acid storage, glucose metabolism, and inflammatory responses [31]. | Alpha-lipoic acid (activates PPAR-α and -γ) [68]; PUFAs [31]. |
Mechanistic Insights from Natural Compounds
Natural compounds often exert their effects via multi-target mechanisms, providing a synergistic approach to immunomodulation.
- Flavonoids and Polyphenols: Compounds like apigenin and luteolin, found in a variety of plants, demonstrate a high degree of interaction with multiple targets, including AKT1, PTGS2, and TP53 [67]. Their anti-inflammatory action is characterized by the modulation of key signaling pathways such as IL-17 and FoxO, which are involved in cytokine production and oxidative stress response [67].
- Organosulfur Compounds: Diallyl trisulfide (from garlic) and allicin exhibit significant immunomodulatory potential, primarily through interaction with a broad range of protein targets, influencing inflammatory gene expression [67].
- Fatty Acids and Derivatives: Alpha-lipoic acid (ALA) exemplifies a pleiotropic modulator. Its reduced form, dihydrolipoic acid (DHLA), chelates heavy metals, regenerates essential antioxidants like glutathione and vitamins C and E, and directly modulates transcription factors [69]. ALA's primary mechanisms include the specific stimulation of Nrf2-dependent gene transcription and the inhibition of NF-κB activity [69] [68]. This dual action enhances the cellular antioxidant defense system while simultaneously suppressing the pro-inflammatory cascade.
Intersection with Alpha-Linolenic Acid (ALA) Metabolism
The metabolism of alpha-linolenic acid (ALA), an essential n-3 polyunsaturated fatty acid (PUFA), is intrinsically linked to inflammatory processes. The conversion of ALA to longer-chain, more bioactive PUFAs like eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) is a critical pathway for generating endogenous anti-inflammatory mediators [31].
Table 2: Key Enzymes and Regulators in ALA Metabolism and Inflammation
| Component | Role in ALA Metabolism & Inflammation | Experimental Findings |
|---|---|---|
| Δ-6 Desaturase (D6D) | Rate-limiting enzyme in the first step of ALA elongation to EPA and DHA [31]. | Gene expression is regulated by PPARα and SREBP-1c and is influenced by the n6/n3 ratio [31]. |
| PPARα | Nuclear transcription factor regulating genes for fatty acid oxidation [31]. | Transcript levels increase with ALA incubation, influencing desaturase expression and ALA conversion efficiency [31]. |
| SREBP-1c | Transcription factor regulating hepatic synthesis of fatty acids [31]. | Involved in the regulation of delta-5 and delta-6 desaturase expression [31]. |
| n6/n3 Fatty Acid Ratio | Represents the dietary balance between pro-inflammatory (n6) and anti-inflammatory (n3) precursors [31]. | An LA/ALA ratio of 1:1 in HepG2 cells resulted in maximum conversion of ALA to EPA (17%) and DHA (0.7%) [31]. Lowering the n6/n3 ratio enhances EPA incorporation into plasma phospholipids [31]. |
The interplay between ALA metabolism and immunomodulation is evident in several areas. Firstly, EPA and DHA give rise to specialized pro-resolving mediators (SPMs) such as resolvins and protectins, which actively promote the resolution of inflammation. Secondly, n-3 PUFAs compete with the n-6 PUFA arachidonic acid (AA) for incorporation into cell membranes and for the enzymatic activity of cyclooxygenases (COX) and lipoxygenases (LOX). This competition leads to a shift in eicosanoid production from pro-inflammatory prostaglandins and leukotrienes (derived from AA) to less inflammatory or anti-inflammatory analogues (derived from EPA) [31]. Furthermore, LCPUFAs like EPA and DHA, or their precursors, act as ligands for nuclear receptors like PPARs, which directly suppress pro-inflammatory gene expression programs [31].
Experimental Protocols and Methodologies
In Vitro Models for Immunomodulatory Assessment
Protocol: Assessing Anti-inflammatory Effects in Cell Cultures (e.g., HepG2, Macrophages)
- Cell Culture & Treatment: Maintain human hepatoma HepG2 cells in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 20% fetal calf serum (FCS), 2% glutamine, and 2% penicillin/streptomycin at 37°C in a 5% CO2 atmosphere [31]. Plate cells at a density of 5 × 10^6 cells in T-25 flasks. For intervention, replace medium with serum-free DMEM and add the compound of interest (e.g., fatty acid mixtures, plant extracts). For fatty acid studies, prepare mixtures like [13C]LA/[13C]ALA in varying ratios (e.g., 9:1, 4:1, 1:1) in sterile bovine serum albumin (BSA) to a final concentration of 100 µM [31].
- Incubation & Harvest: Incubate cells for a predetermined period (e.g., 24 hours). Harvest cells using accutase, wash with PBS, and centrifuge at 500 × g. The cell pellet can be stored at -75°C for subsequent analysis [31].
- Downstream Analysis:
- Lipid & Metabolite Analysis: Extract lipids using a modified Folch extraction (chloroform:methanol, 2:1 v/v) [31]. Analyze fatty acid composition or metabolite profiles using Gas Chromatography-Mass Spectrometry (GC-MS) or Liquid Chromatography-Mass Spectrometry (LC-MS/MS).
- Gene Expression: Isolate total RNA and perform Reverse Transcription-Polymerase Chain Reaction (RT-PCR) to quantify transcript levels of genes of interest (e.g., D5D, D6D, PPARα, SREBP-1c, pro-inflammatory cytokines) [31].
- Protein Analysis: Use Western Blotting or ELISA to measure protein levels of cytokines (e.g., IL-1β, IL-6, TNF-α, IL-10) or phosphorylated signaling proteins (e.g., p-NF-κB, p-IκB) [70].
Advanced Metabolomics for Biomarker Discovery
The GNPS2 (Global Natural Products Social Molecular Networking) environment provides a powerful, community-driven platform for conducting untargeted metabolomics in drug metabolism and biomarker discovery [71].
Protocol: Metabolite Identification and Workflow using GNPS2
- Mass Spectrometry Data Acquisition: Analyze biological samples (plasma, CSF, cell homogenates) using Liquid Chromatography–Quadrupole Time-of-Flight Tandem Mass Spectrometry (LC–QTOF-MS/MS) to obtain MS/MS spectral data [71].
- Data Processing and Molecular Networking: Process raw MS data using feature detection tools (e.g., MZmine). Submit the resulting MS/MS spectra to GNPS2 to create a Feature-Based Molecular Network (FBMN). This workflow clusters molecules based on MS/MS spectral similarity, allowing for the discovery of candidate drug metabolites and related endogenous metabolites without prior knowledge [71].
- Statistical Analysis and Annotation: Utilize the FBMN-STATS tool within GNPS2 to perform univariate and multivariate statistical analyses between sample groups (e.g., control vs. treated) [71]. Annotate metabolites of interest by integrating in-silico tools such as SIRIUS (for predicting molecular formulas and fragmentation trees) and Chemwalker (for structural prediction) [71].
- Repository-Level Validation: Use the MASST (Mass Spectrometry Search Tool) to search the MS/MS spectra of putatively identified metabolites against all public metabolomics data within the GNPS ecosystem, supporting the identification through "reverse metabolomics" [71].
Figure 1: GNPS2 Metabolomics Analysis Workflow. This diagram outlines the key steps in a comprehensive metabolomics workflow using the GNPS2 platform for biomarker discovery and drug metabolism studies.
The Scientist's Toolkit: Essential Research Reagents
Successful investigation into anti-inflammatory and immunomodulatory mechanisms relies on a suite of specialized reagents and tools.
Table 3: Key Research Reagent Solutions for Immunomodulation Studies
| Reagent / Tool | Function & Application | Specific Examples / Notes |
|---|---|---|
| Cell Line Models | In vitro systems for mechanistic studies and high-throughput screening. | HepG2 (Human hepatoma): For fatty acid metabolism, lipid manipulation, and transcriptome studies [31]. Primary Immune Cells (e.g., Monocytes/Macrophages): For direct assessment of cytokine release and phagocytosis. |
| Bioactive Compounds | Standardized compounds for treatment interventions and pathway modulation. | Alpha-Lipoic Acid: For studying Nrf2/NF-κB crosstalk and antioxidant pathways [69] [68]. Apigenin, Luteolin, Silibinin: Flavonoids for multi-target network pharmacology studies [67]. PUFA Mixtures (e.g., [13C]ALA/[13C]LA): For tracing metabolic flux and studying n6/n3 ratio effects [31]. |
| Analytical Platforms | Instruments for identifying and quantifying metabolites, lipids, and proteins. | LC-QTOF-MS/MS: High-resolution mass spectrometry for untargeted metabolomics and molecular networking via GNPS2 [71]. GC-MS: For precise fatty acid methyl ester (FAME) analysis and stable isotope tracing [31]. |
| Software & Databases | Bioinformatics tools for data analysis, network construction, and target prediction. | GNPS2 Platform: For molecular networking, statistics, and repository mining [71]. STITCH Database: For predicting chemical-protein interactions [67]. Cytoscape: For visualization of complex constituent-target-pathway networks [67]. |
| 6-Aminopenicillanic acid | 6-Aminopenicillanic acid, CAS:551-16-6, MF:C8H12N2O3S, MW:216.26 g/mol | Chemical Reagent |
| 7-APRA | 7-APRA, CAS:107937-01-9, MF:C10H12N2O3S, MW:240.28 g/mol | Chemical Reagent |
Signaling Pathways and Workflow Visualizations
Understanding the intricate interplay between key signaling pathways is fundamental to grasping the molecular basis of immunomodulation. The following diagram synthesizes the core mechanisms of action for several key modulators, such as alpha-lipoic acid and n-3 PUFAs, illustrating their impact on the central regulators NF-κB and Nrf2.
Figure 2: Core Anti-inflammatory and Antioxidant Signaling Pathways. This diagram illustrates the dual mechanism of action of compounds like ALA and n-3 PUFAs, showing inhibition of the pro-inflammatory NF-κB pathway and activation of the cytoprotective Nrf2-ARE pathway.
Neuroprotective Effects and Central Nervous System Applications
Alpha-linolenic acid (ALA), an essential n-3 polyunsaturated fatty acid, demonstrates significant neuroprotective potential through multiple mechanistic pathways. This whitepaper synthesizes current evidence on ALA's central nervous system applications, highlighting its anti-inflammatory, antioxidant, and pro-degradation effects against amyloid-beta proteins. We present comprehensive experimental methodologies, quantitative data analyses, and molecular pathway visualizations to guide research and drug development. Emerging clinical evidence suggests ALA supplementation increases brain-derived neurotrophic factor (BDNF) levels in humans, supporting its therapeutic potential for neurodegenerative conditions including Alzheimer's disease. The integration of ALA metabolism research with neurodegenerative disease mechanisms offers promising avenues for developing novel neuroprotective interventions.
Alpha-linolenic acid (ALA) is an essential C-18 n-3 polyunsaturated fatty acid (PUFA) that must be obtained through dietary sources as humans lack the desaturase enzymes required for its synthesis [56]. ALA serves as a precursor to longer-chain n-3 PUFAs including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), though conversion rates in humans are limited, particularly for DHA [56]. Despite conversion limitations, substantial evidence indicates that ALA itself exerts direct neuroprotective effects through multiple molecular mechanisms.
Research advancements in neurodegenerative diseases reveal common pathogenic mechanisms across conditions. Alzheimer's disease (AD) and Parkinson's disease (PD), once considered distinct entities, share considerable clinical and neuropathological overlap, with many patients exhibiting features of both conditions [72]. This overlap suggests common neurodegenerative mechanisms that may be targeted by neuroprotective compounds like ALA. Both diseases involve toxic protein aggregates—amyloid-beta and tau in AD, and alpha-synuclein in PD—that damage brain cells and disrupt neural function [73]. ALA demonstrates protective effects against several of these pathological processes.
The neuroprotective potential of ALA extends beyond its role as a structural membrane component. ALA is metabolized to various oxylipins through lipoxygenase (LOX), cyclooxygenase (COX), and cytochrome P450 (CYP450) pathways, leading to bioactive metabolites including 9- and 13-hydroxy-octadecatrienoic acids (9-HOTrE and 13-HOTrE) [56]. These metabolites exhibit immunomodulating effects that may contribute to ALA's neuroprotective properties in various experimental models.
Mechanisms of Neuroprotection
Anti-inflammatory Pathways
Neuroinflammation represents a critical pathological process in numerous neurodegenerative diseases. ALA demonstrates significant anti-inflammatory effects through modulation of glial cell activity and inflammatory mediator production:
Suppression of glial-mediated inflammation: In C6 glial cells exposed to amyloid-beta (Aβ25-35), ALA treatment markedly attenuated the overproduction of nitric oxide (NO) and pro-inflammatory cytokines including interleukin (IL)-6 and tumor necrosis factor-α (TNF-α) [74]. This anti-inflammatory effect was mediated through down-regulation of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) at both protein and mRNA expression levels [74].
Inhibition of nuclear factor kappa B (NF-κB) signaling: The reduction in inflammatory mediators is associated with suppressed NF-κB activation, a master regulator of inflammation. Through this mechanism, ALA interrupts the vicious cycle of chronic neuroinflammation that characterizes progressive neurodegenerative conditions.
Modulation of peripheral inflammation: In human studies, ALA supplementation has been associated with reduced levels of C-reactive protein (CRP), a systemic inflammatory biomarker linked to cardiovascular and neurodegenerative diseases [56]. This systemic anti-inflammatory effect may indirectly benefit central nervous system health by reducing peripheral inflammatory signals that can exacerbate neuroinflammation.
Antioxidant Activities and Nrf-2 Pathway Activation
Oxidative stress represents another fundamental mechanism in neurodegenerative pathology. ALA activates endogenous antioxidant defense systems through the following mechanisms:
Nrf-2/HO-1 pathway activation: In C6 glial cells, ALA treatment inhibited reactive oxygen species (ROS) generation induced by Aβ25-35 through enhancement of nuclear factor-erythroid 2-related factor-2 (Nrf-2) protein levels and subsequent induction of heme-oxygenase-1 (HO-1) expression in a dose- and time-dependent manner [74]. The Nrf-2/HO-1 pathway serves as a critical cellular defense mechanism against oxidative stress.
Reduction of lipid peroxidation: While ALA supplementation increases plasma malondialdehyde (MDA) levels, indicating enhanced lipid peroxidation [75], this may reflect increased PUFA metabolism rather than pathological oxidation. The net effect of ALA on oxidative balance appears beneficial, as demonstrated by protection against Aβ-mediated oxidative damage.
The following diagram illustrates the primary neuroprotective mechanisms of ALA:
Enhancement of Amyloid-Beta Clearance
In Alzheimer's disease models, ALA demonstrates unique effects on amyloid-beta pathology through enhanced degradation mechanisms:
Upregulation of amyloid-degrading enzymes: ALA treatment significantly increased the protein expression of neprilysin and insulin-degrading enzyme (IDE), both of which contribute to the degradation of amyloid-beta peptides [74]. This enhanced clearance mechanism represents a direct approach to reducing the amyloid burden characteristic of Alzheimer's pathology.
Reduction of amyloid-induced toxicity: By promoting amyloid-beta clearance, ALA protects against Aβ-mediated neurotoxicity in glial cells, with studies demonstrating increased cell viability in Aβ-exposed cultures treated with ALA [74].
Neurotrophic Effects and BDNF Enhancement
ALA influences neurotrophic factor signaling, particularly brain-derived neurotrophic factor (BDNF), which plays crucial roles in neuronal survival, differentiation, and synaptic plasticity:
Increased BDNF expression: Oral ALA supplementation significantly increases plasma BDNF levels in healthy adult humans, with more pronounced effects observed in women compared to men [75]. BDNF promotes neurogenesis, neuronal survival, and synaptogenesis through activation of tropomyosin receptor kinase (TrkB) receptors.
Promotion of neurogenesis: Preclinical studies demonstrate that ALA administration induces neurogenesis in the hippocampus, promotes neural stem cell proliferation, and enhances synaptogenesis [75]. These effects contribute to neural repair and maintenance of cognitive function.
Quantitative Evidence and Experimental Data
Human Clinical Studies
Table 1: Effects of ALA Supplementation on Neuroprotective Biomarkers in Human Studies
| Study Population | ALA Dose & Duration | BDNF Changes | MDA Changes | Inflammatory Marker Changes | Reference |
|---|---|---|---|---|---|
| 30 healthy adults (15M, 15F) | 1.5 g/day (3×500 mg capsules) for 1 week | Significant increase (P<0.05); greater in women | Significant increase (P<0.05) | Not measured | [75] |
| Mixed populations (healthy, T2D, dyslipidemia) | 1.9-10 g/day for 2-12+ weeks | Not measured | Not measured | ↓ C-reactive protein (CRP) in CKD patients | [56] |
In Vitro Experimental Findings
Table 2: Neuroprotective Effects of ALA in C6 Glial Cell Models Exposed to Amyloid-Beta
| Experimental Parameter | Aβ25-35 Exposure Alone | Aβ25-35 + ALA Treatment | Significance & Notes |
|---|---|---|---|
| Cell viability | Decreased | Increased | Dose-dependent protection |
| NO production | Overproduction | Markedly attenuated | Downregulation of iNOS |
| Pro-inflammatory cytokines | IL-6 and TNF-α release | Significant reduction | Downregulation of COX-2 |
| ROS generation | Induced | Inhibited | Via Nrf-2/HO-1 activation |
| Neprilysin expression | Baseline | Increased | Enhanced Aβ degradation |
| IDE expression | Baseline | Increased | Enhanced Aβ degradation |
Experimental Protocols and Methodologies
In Vitro Assessment of Neuroprotective Effects
Cell Culture Model: C6 glial cell line for neuroinflammation studies [74]
Amyloid-Beta Preparation:
- Prepare Aβ25-35 peptide solution at 1 mM concentration in sterile distilled water
- Incubate at 37°C for 7 days to allow aggregation
- Store at -20°C until use
ALA Treatment Protocol:
- Prepare ALA stock solution in appropriate vehicle (e.g., DMSO or ethanol)
- Dilute to working concentrations in culture medium (typical range: 10-100 μM)
- Pre-treat cells with ALA for 2-24 hours before Aβ25-35 exposure
- Co-incubate ALA with Aβ25-35 (50 μM) for 24 hours
Assessment Methods:
- Cell viability: MTT assay or LDH release measurement
- Nitric oxide production: Griess reaction for nitrite accumulation
- Cytokine measurements: ELISA for IL-6 and TNF-α levels
- Protein expression: Western blot for iNOS, COX-2, Nrf-2, HO-1, neprilysin, and IDE
- mRNA expression: RT-qPCR for inflammatory mediators
- ROS measurement: DCFH-DA fluorescence assay
Clinical Assessment Protocol
Human Supplementation Studies [75]:
- Participant selection: Healthy adults with BMI <30, no underlying diseases
- Baseline blood collection: 5 mL in EDTA-containing tubes
- ALA supplementation: 1.5 g/day (divided doses) for 7 days
- Post-treatment blood collection: Day 8
- Plasma separation: Centrifuge at 3000 rpm for 10 minutes
- Biomarker assessment:
- BDNF: Emax ImmunoAssay System
- MDA: Colorimetric assay kit
Research Reagent Solutions
Table 3: Essential Research Reagents for Investigating ALA Neuroprotection
| Reagent/Cell Line | Specific Example | Research Application | Key Findings with ALA |
|---|---|---|---|
| C6 glial cells | Rat glioma cell line | Neuroinflammation models | ALA reduces Aβ-induced inflammation and oxidative stress [74] |
| Aβ25-35 peptide | Aggregated form | Alzheimer's disease model | ALA enhances cell viability and reduces toxicity [74] |
| BDNF Emax ImmunoAssay | Promega Corporation | BDNF quantification in plasma | ALA supplementation increases BDNF levels in humans [75] |
| MDA colorimetric assay | Oxford Biomedical Research | Lipid peroxidation measurement | ALA supplementation increases MDA in human plasma [75] |
| Primary neuronal cultures | Cortical or hippocampal neurons | Neuronal survival and function | ALA metabolites show immunomodulating effects [56] |
Integration with Neurodegenerative Disease Research
The neuroprotective properties of ALA must be considered within the broader context of neurodegenerative disease mechanisms and current therapeutic development:
Overlap in Neurodegenerative Pathology: The considerable overlap between Alzheimer's and Parkinson's diseases suggests common neurodegenerative mechanisms that may respond to similar therapeutic approaches [72]. Both conditions involve protein aggregation, neuroinflammation, and oxidative stress—processes that ALA targets through its multiple mechanisms of action.
Current Drug Development Landscape: The Alzheimer's drug development pipeline currently includes 138 drugs across 182 clinical trials, with biological disease-targeted therapies comprising 30% and small molecule therapies accounting for 43% of the pipeline [76]. ALA-based approaches would fall into the small molecule category with potential disease-modifying properties.
Therapeutic Targeting of Protein Aggregation: Recent advances in Alzheimer's treatment include drugs that reduce amyloid-beta proteins, suggesting that similar approaches targeting toxic protein aggregates may be effective for other neurodegenerative conditions [73]. ALA's ability to enhance amyloid-beta clearance through upregulation of degrading enzymes positions it as a potential modifier of protein aggregation pathology.
Biomarker Development: Biomarkers serve as primary outcomes in 27% of active Alzheimer's trials [76], highlighting the importance of objective measures in neurodegenerative disease research. ALA's effects on BDNF, inflammatory markers, and oxidative stress parameters provide potential biomarker signatures for assessing therapeutic response.
Alpha-linolenic acid demonstrates multifaceted neuroprotective effects through anti-inflammatory, antioxidant, amyloid-modifying, and neurotrophic mechanisms. The experimental evidence from cellular models and human studies supports its potential relevance for central nervous system disorders characterized by neuroinflammation, oxidative stress, and protein aggregation pathology.
Future research should prioritize:
- Dose-response characterization in relevant disease models
- Combination therapies with existing neuroprotective approaches
- Biomarker validation for target engagement and efficacy assessment
- Formulation strategies to enhance CNS bioavailability
- Population studies in early-stage neurodegenerative diseases
The integration of ALA research with broader neurodegenerative disease mechanisms offers promising avenues for developing novel interventions that target fundamental pathological processes shared across multiple conditions. As drug development efforts advance, ALA and its metabolites represent compelling candidates for further investigation as potential disease-modifying agents.
ALA in Cancer Prevention and Metabolic Syndrome Management
Alpha-lipoic acid (ALA), a naturally occurring dithiol compound synthesized in mitochondria, demonstrates significant potential in managing chronic diseases characterized by oxidative stress. This whitepaper provides a comprehensive technical analysis of ALA's dual mechanisms in cancer prevention and metabolic syndrome (MetS) management, focusing on its antioxidant and pro-oxidative properties, molecular pathways, and clinical applications. We synthesize current research on ALA's multifaceted functions, including its roles in redox regulation, inflammatory pathway modulation, insulin sensitization, and energy metabolism. Experimental protocols, mechanistic diagrams, and research reagent solutions are presented to facilitate further investigation and drug development targeting ALA-associated pathways.
Alpha-lipoic acid (1,2-dithiolane-3-pentanoic acid) and its reduced form, dihydrolipoic acid (DHLA), function as powerful antioxidants with unique amphipathic properties, enabling distribution in both hydrophilic and lipophilic environments [77]. Since its discovery in 1937, ALA has garnered significant research interest due to its simple structure and complex biological functions [4]. As an essential cofactor for mitochondrial enzyme complexes including pyruvate dehydrogenase, branched-chain 2-ketoacid dehydrogenase, and 2-ketoglutarate dehydrogenase, ALA plays fundamental roles in cellular energy metabolism [77]. The compound's therapeutic potential extends beyond its metabolic functions to encompass antioxidant, anti-inflammatory, and immunomodulatory properties, positioning it as a promising agent for addressing the intersecting pathophysiological mechanisms of cancer and metabolic syndrome [77] [4].
ALA in Cancer Prevention
Dual Antioxidant and Pro-Oxidative Mechanisms
ALA exhibits a paradoxical dual nature in cancer contexts, functioning as an antioxidant in normal cells while demonstrating pro-oxidative capabilities in the unique redox environment of cancer cells [77]. This selective toxicity toward malignant cells represents a promising therapeutic avenue:
Antioxidant Protection in Normal Cells: ALA and DHLA directly quench various reactive species including ROS, reactive nitrogen species, hydroxyl radicals, hypochlorous acid, and singlet oxygen [77]. The ALA/DHLA system exhibits a high redox potential (320 mV), enabling more effective maintenance of the reductive state compared to the endogenous glutathione system (240 mV) [77]. Additionally, ALA provides indirect antioxidant protection through metal chelation and regeneration of endogenous antioxidants including vitamin E, vitamin C, and glutathione [77].
Pro-Oxidative Effects in Cancer Cells: Research indicates that ALA's mechanism in cancer cells is distinct from that observed in normal cells, exhibiting pro-oxidative properties that selectively target malignant cells [77]. The compound promotes oxidation in the cancer microenvironment, inhibits pro-cancer pathways, and activates tumor suppressor genes, potentially impacting various stages of cancer development [77].
Molecular Pathways and Mechanisms
ALA influences multiple carcinogenic and anti-carcinogenic pathways through several interconnected mechanisms:
Figure 1: ALA modulates multiple signaling pathways in cancer cells, exhibiting both antioxidant and pro-oxidative effects depending on cellular context.
Inflammatory Pathway Modulation: ALA acts as an antagonist of various inflammatory pathways, inhibiting NF-κB mediated inflammation and reducing pro-inflammatory cytokines including TNF-α and IL-6 [77]. Simultaneously, ALA enhances the activity of the anti-inflammatory protein nuclear factor erythroid 2-related factor 2 (Nrf2), thereby reducing tissue damage and creating an unfavorable microenvironment for cancer progression [77].
Hypoxia-Inducible Factor Regulation: ALA demonstrates inhibitory effects on hypoxia-inducible factors (HIFs), which are frequently activated in the tumor microenvironment and contribute to angiogenesis and metastasis [77]. By modulating HIF activity, ALA potentially disrupts tumor adaptation to hypoxic conditions.
Effects on Cancer Stem Cells and EMT: Emerging research indicates ALA's potential role in targeting cancer stem cells (CSCs) and inhibiting epithelial-mesenchymal transition (EMT), critical processes in tumor initiation, progression, and therapeutic resistance [77].
Anti-Infective and Anti-Carcinogenic Effects
Chronic infections contribute to approximately 13% of cancer diagnoses worldwide [77]. ALA exerts multiple beneficial effects in infection-related carcinogenesis:
Pathogen Restriction: ALA helps maintain redox homeostasis, preventing the redox state from being skewed in favor of pathogen proliferation [77]. The compound regenerates depleted glutathione during viral replication and internalization, enhancing immune system function and creating an environment less conducive to pathogen persistence [77].
Immune System Support: ALA can increase TCA cycle activity, enhancing ATP production and providing energy for immune activities and healing [77]. The compound's inflammation suppression properties indirectly enhance immune system function, preventing hypersensitive reactions such as cytokine storms that can contribute to chronic tissue damage and carcinogenesis [77].
ALA in Metabolic Syndrome Management
Metabolic Syndrome Pathophysiology and ALA Mechanisms
Metabolic syndrome represents a constellation of metabolic abnormalities including central obesity, dyslipidemia, hypertension, and insulin resistance, significantly increasing cardiovascular disease and type 2 diabetes risk [78] [79]. The global prevalence of MetS is approximately 25%, with variations across populations and age groups [79]. ALA addresses multiple pathophysiological components of MetS through interconnected mechanisms:
Figure 2: ALA targets multiple pathological components of metabolic syndrome through integrated mechanisms involving insulin sensitivity, inflammation, oxidative stress, and endothelial function.
Insulin Sensitization: ALA enhances insulin-mediated glucose disposal through multiple mechanisms, including activation of insulin receptor substrate-1 (IRS-1) and downstream signaling components, translocation of GLUT4 transporters to the cell membrane, and modulation of mitochondrial function in insulin-sensitive tissues [4].
Oxidative Stress Reduction: As a potent antioxidant, ALA mitigates the chronic oxidative stress characteristic of MetS, reducing lipid peroxidation, protein carbonylation, and DNA oxidation that contribute to insulin resistance and endothelial dysfunction [4].
Inflammatory Pathway Modulation: ALA suppresses pro-inflammatory signaling pathways, particularly NF-κB activation, reducing the production of inflammatory cytokines (TNF-α, IL-6) and chemokines that promote insulin resistance and atherosclerotic processes [77] [4].
Endothelial Function Improvement: ALA enhances nitric oxide-mediated endothelium-dependent vasodilation, improving microcirculation and counteracting the endothelial dysfunction that underlies hypertension and vascular complications in MetS [4].
Clinical Evidence and Clinical Applications
ALA demonstrates particularly strong evidence in diabetic neuropathy management, enhancing nitric oxide-mediated vasodilation and improving microcirculation in patients with diabetic polyneuropathy [4]. Clinical studies support ALA's benefits across multiple MetS components:
Table 1: Clinical Evidence for ALA in Metabolic Syndrome Management
| MetS Component | ALA Dosage | Study Duration | Key Outcomes | References |
|---|---|---|---|---|
| Diabetic Neuropathy | 600 mg IV daily | 3 weeks | Improved neuropathic symptoms and deficits | [4] |
| Insulin Resistance | 300-600 mg oral daily | 3-6 months | Improved insulin sensitivity | [4] |
| Oxidative Stress Markers | 300-600 mg oral daily | 1-6 months | Reduced lipid peroxidation, increased glutathione | [4] |
| Endothelial Function | 300-600 mg oral daily | 3-6 months | Improved flow-mediated dilation | [4] |
Experimental Protocols and Methodologies
Assessing ALA Antioxidant Capacity In Vitro
Objective: Evaluate ALA and DHLA direct free radical scavenging capacity using established antioxidant assays.
Reagents and Equipment:
- ALA and DHLA standards
- DPPH (2,2-diphenyl-1-picrylhydrazyl) solution
- ABTS (2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) solution
- Phosphate buffer (pH 7.4)
- UV-Vis spectrophotometer
- Microplate reader
- Incubator
Procedure:
- Prepare ALA and DHLA solutions in phosphate buffer at concentrations ranging from 10-500 μM.
- For DPPH assay: Mix 100 μL of each ALA/DHLA solution with 900 μL of 0.1 mM DPPH in methanol.
- Incubate for 30 minutes in darkness at room temperature.
- Measure absorbance at 517 nm against methanol blank.
- For ABTS assay: Generate ABTS radical cation by reacting 7 mM ABTS with 2.45 mM potassium persulfate for 12-16 hours.
- Dilute ABTS solution to absorbance of 0.70 ± 0.02 at 734 nm.
- Mix 10 μL ALA/DHLA solutions with 990 μL diluted ABTS solution.
- Incubate for 6 minutes and measure absorbance at 734 nm.
- Calculate percentage radical scavenging activity compared to untreated control.
Data Analysis: Calculate IC50 values (concentration providing 50% radical scavenging) using non-linear regression analysis. Compare potency to standard antioxidants (ascorbic acid, Trolox).
Evaluating ALA Effects on Inflammatory Signaling
Objective: Investigate ALA modulation of NF-κB signaling pathway in cultured macrophages.
Cell Culture and Treatment:
- Maintain RAW 264.7 macrophages in DMEM with 10% FBS at 37°C, 5% CO2.
- Pre-treat cells with ALA (50-500 μM) for 2 hours.
- Stimulate with LPS (100 ng/mL) for 4-24 hours.
NF-κB Activation Assessment:
- Nuclear extract preparation using commercial kits
- EMSA (electrophoretic mobility shift assay) for NF-κB DNA binding
- Western blot for IκBα degradation and NF-κB p65 nuclear translocation
- Immunofluorescence for p65 subcellular localization
Cytokine Measurement:
- Collect culture supernatants after LPS stimulation
- Quantify TNF-α, IL-6 using ELISA kits
- RNA extraction and qRT-PCR for cytokine gene expression
Statistical Analysis: One-way ANOVA with post-hoc Tukey test, significance at p<0.05.
Research Reagent Solutions
Table 2: Essential Research Reagents for ALA Investigations
| Reagent/Category | Specific Examples | Research Applications | Key Functions |
|---|---|---|---|
| ALA Formulations | R-ALA (R-enantiomer), Na-R-ALA (sodium salt) | Bioavailability studies, enantiomer-specific effects | Enhanced cellular uptake, improved pharmacokinetics |
| Antibody Panels | Anti-NF-κB p65, anti-IκBα, anti-phospho-IκBα | Inflammatory signaling studies | Detection of pathway activation and inhibition |
| Oxidative Stress Assays | DCFDA, DHE, Lipid Peroxidation (MDA) Assay | Antioxidant/pro-oxidant activity assessment | ROS detection, lipid peroxidation quantification |
| Metabolic Assays | Glucose Uptake Assay, Insulin Signaling Panel | Insulin resistance mechanisms | Glucose metabolism assessment, signaling pathway analysis |
| Molecular Biology Tools | NF-κB Reporter Cell Lines, Nrf2 Reporter Assay | Pathway-specific activity screening | High-throughput screening of ALA effects on transcription factors |
Alpha-lipoic acid represents a promising multifaceted therapeutic agent with significant potential in cancer prevention and metabolic syndrome management. Its unique dual functionality as both an antioxidant in normal cells and a pro-oxidative agent in cancer cells, combined with its anti-inflammatory and insulin-sensitizing properties, positions ALA as a valuable candidate for further drug development. The experimental protocols and research reagents outlined in this whitepaper provide a foundation for advancing our understanding of ALA's mechanisms and therapeutic applications. Future research should focus on optimizing ALA formulations for enhanced bioavailability, conducting well-designed clinical trials across different disease stages, and exploring synergistic combinations with conventional therapeutics to maximize clinical benefits while minimizing adverse effects.
Challenges and Optimization Strategies in ALA Research
The endogenous synthesis of docosahexaenoic acid (DHA) from its plant-derived precursor, alpha-linolenic acid (ALA), is characterized by notably low efficiency in humans. This comprehensive review examines the multifaceted biological constraints governing this conversion process, including competitive enzyme kinetics, genetic polymorphisms, gender-specific differences, and alternative metabolic fates of ALA. We present quantitative data from stable isotope studies, detailed experimental methodologies for investigating this pathway, and visualizations of the underlying metabolic processes. The evidence synthesized herein supports the consideration of pre-formed DHA as a conditionally essential nutrient in specific populations, with important implications for nutritional science and therapeutic development.
Alpha-linolenic acid (ALA, 18:3n-3), the essential omega-3 fatty acid derived from plant sources, serves as the metabolic precursor for the synthesis of long-chain polyunsaturated fatty acids (LC-PUFAs), including eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3) [28] [6]. These LC-PUFAs play critical roles in neurological development, visual function, and inflammatory regulation [6]. Despite the theoretical pathway for endogenous DHA synthesis, substantial clinical and experimental evidence reveals severe biological constraints that limit the conversion efficiency in humans, making ALA an inadequate source for maintaining optimal DHA status [80] [81]. This whitepaper examines the biochemical, genetic, and physiological factors governing this limited conversion, providing researchers and drug development professionals with a comprehensive analysis of this metabolic challenge.
Metabolic Pathways and Enzymatic Constraints
The ALA to DHA Biosynthetic Pathway
The conversion of ALA to DHA occurs through a series of elongation and desaturation reactions primarily in the liver [6]. This pathway begins with Δ6-desaturation of ALA to stearidonic acid (18:4n-3), followed by elongation to eicosatetraenoic acid (20:4n-3), Δ5-desaturation to EPA (20:5n-3), elongation to docosapentaenoic acid (DPA; 22:5n-3), and further elongation to 24:5n-3. This intermediate is then desaturated by Δ6-desaturase to 24:6n-3, and finally undergoes β-oxidation in peroxisomes to produce DHA (22:6n-3) [82]. This final retroconversion step, known as the Sprecher pathway, adds further complexity and potential for inefficiency in DHA synthesis [82].
It is important to note that alternative DHA biosynthesis pathways exist in certain marine microorganisms. Some thraustochytrids utilize a polyunsaturated fatty acid synthase (PUFA-S) system for anaerobic DHA synthesis, while others employ a complete aerobic pathway involving elongases (ELO) and desaturases (DES), including a Δ4-desaturase that directly converts n-3 DPA to DHA [83]. However, humans lack this direct Δ4-desaturase capability, necessitating the more circuitous Sprecher pathway [82].
Enzyme Competition and Inhibitory Factors
A primary constraint in the ALA to DHA conversion pathway is the competition for enzymes between the omega-3 and omega-6 PUFA families. Both ALA and linoleic acid (LA; 18:2n-6) compete for the same Δ6-desaturase enzyme in the initial rate-limiting step of their respective conversion pathways [28] [6]. The typical Western diet, characterized by an LA:ALA ratio of approximately 10:1, creates a competitive environment strongly favoring LA metabolism over ALA conversion [28] [81]. This high dietary LA intake significantly inhibits the conversion of ALA to its long-chain metabolites by monopolizing the desaturase enzymes [84].
Figure 1: Competitive Inhibition in ALA to DHA Conversion Pathway. The metabolic pathways for omega-3 (yellow) and omega-6 (red) polyunsaturated fatty acids share the same enzyme systems, creating competitive inhibition that limits DHA production, particularly with high dietary linoleic acid intake.
Quantitative Assessment of Conversion Efficiency
Human Conversion Efficiency Studies
Stable isotope tracer studies have provided precise measurements of ALA to DHA conversion efficiency in humans. These investigations reveal consistently low conversion rates, with significant variability based on gender, genetic factors, and dietary composition.
Table 1: ALA to DHA Conversion Efficiencies in Human Studies
| Study Population | ALA to EPA Conversion | ALA to DHA Conversion | Key Findings | Citation |
|---|---|---|---|---|
| Healthy Young Men | ~8% | 0%-4% | Limited conversion, especially for DHA | [6] |
| Healthy Young Women | ~21% | ~9% | Significantly higher conversion than men | [6] |
| Lactating Women | No significant increase | No significant increase | 10.7 g/day ALA failed to increase milk DHA | [80] |
| Mixed Population | <5% overall | 0.5%-5% overall | High individual variability observed | [84] |
The gender disparity in conversion efficiency is particularly noteworthy, with women demonstrating approximately 2-3 times greater conversion capacity than men, potentially mediated by estrogen effects [6]. This enhanced conversion efficiency in women may represent a biological adaptation to support fetal and infant neurological development during pregnancy and lactation.
Impact of High-ALA Interventions
Long-term intervention studies with high ALA intake further demonstrate the limitations of conversion efficiency. A 12-week high-ALA intervention (14.0 ± 0.45 g/day) in subjects with low EPA and DHA status revealed that while EPA concentrations in red blood cells significantly increased from 6.13 ± 0.51 μg/mL to 11.0 ± 0.64 μg/mL, DHA concentrations unexpectedly decreased from 41.0 ± 1.93 μg/mL to 30.4 ± 1.09 μg/mL [84]. This paradoxical decrease in DHA levels despite high ALA supplementation highlights the complex regulation of DHA homeostasis and suggests potential diversion of ALA into other metabolic pathways.
Table 2: Red Blood Cell PUFA Changes During 12-Week High-ALA Intervention
| Time Point | ALA Concentration (μg/mL) | EPA Concentration (μg/mL) | DHA Concentration (μg/mL) | ΣEPA + DHA (% of total FA) |
|---|---|---|---|---|
| Baseline (Week 0) | 1.44 ± 0.10 | 6.13 ± 0.51 | 41.0 ± 1.93 | 4.63 ± 0.19 |
| Week 1 | 4.65 ± 0.22 | 7.33 ± 0.33 | 37.0 ± 1.32 | 4.67 ± 0.16 |
| Week 3 | 5.47 ± 0.23 | 8.38 ± 0.42 | 36.1 ± 1.37 | 4.61 ± 0.13 |
| Week 6 | 6.25 ± 0.24 | 10.9 ± 0.67 | 35.1 ± 1.06 | 4.73 ± 0.15 |
| Week 12 | 5.80 ± 0.28 | 11.0 ± 0.64 | 30.4 ± 1.09 | 4.52 ± 0.11 |
Key Experimental Approaches and Methodologies
Stable Isotope Tracer Protocols
The gold standard for assessing in vivo conversion efficiency employs stable isotope-labeled ALA (typically 13C- or 2H-labeled) with subsequent monitoring of labeled EPA and DHA appearance in blood compartments.
Protocol: Stable Isotope Tracer Study Design
- Participant Preparation: Subjects maintain controlled diets with defined PUFA content for 1-2 weeks pre-study to standardize background fatty acid status
- Tracer Administration: Single oral dose of 0.5-1 mg/kg body weight of isotope-labeled ALA administered with a standard meal
- Blood Sampling: Serial blood samples collected at baseline, 2, 4, 6, 8, 12, 24, 48, 72, and 96 hours post-administration
- Sample Processing: Plasma and erythrocyte fractions separated by centrifugation; lipid extraction via Folch or Bligh-Dyer methods
- Fatty Acid Analysis: Preparation of fatty acid methyl esters (FAMEs) followed by gas chromatography-mass spectrometry (GC-MS) or gas chromatography-combustion-isotope ratio mass spectrometry (GC-C-IRMS)
- Kinetic Modeling: Compartmental modeling to calculate conversion rates and fractional conversion efficiencies [80]
Dietary Intervention Studies
Long-term dietary modification studies provide complementary data on steady-state changes in PUFA status under controlled conditions.
Protocol: 12-Week High-ALA Intervention
- Participant Selection: Recruit subjects with low baseline EPA+DHA status (Omega-3 Index <4%)
- Dietary Modification: Provide all meals or specific food items (e.g., linseed oil, walnuts) to achieve target ALA intake (typically 10-15 g/day)
- Compliance Monitoring: Food diaries, 24-hour recalls, and plasma phospholipid ALA measurements
- Endpoint Assessment: Red blood cell fatty acid composition at multiple time points (weeks 0, 1, 3, 6, 12)
- Oxylipin Profiling: LC-MS-based targeted metabolomics of plasma oxylipins to monitor downstream metabolic effects [84]
Genetic and Physiological Modifiers
Genetic Polymorphisms
Common genetic variants in the fatty acid desaturase (FADS) gene cluster account for approximately 30% of the variability in LC-PUFA levels among individuals [6]. Specific haplotypes (e.g., FADS haplotype D) are associated with increased Δ5- and Δ6-desaturase activity and higher conversion rates of ALA to EPA and DHA [6]. These genetic differences may explain the substantial interindividual variability observed in conversion efficiency studies and suggest a personalized approach to omega-3 nutritional recommendations.
Alternative Metabolic Fates of ALA
Beyond conversion to LC-PUFAs, ALA undergoes several alternative metabolic processes that compete with DHA synthesis:
- β-oxidation: In humans, 16-34% of ingested ALA is oxidized for energy within 12-24 hours, making this a significant metabolic fate that diverts ALA from elongation/desaturation pathways [81]
- Carbon recycling: A portion of ALA carbon skeletons are recycled for de novo lipogenesis in various tissues [81]
- Direct incorporation: ALA can be directly incorporated into tissue phospholipids and triglycerides without conversion [28]
Figure 2: Metabolic Fates of Dietary Alpha-Linolenic Acid. The majority of ingested ALA is partitioned toward energy production (β-oxidation) and direct tissue incorporation rather than conversion to long-chain omega-3 PUFAs, with DHA synthesis representing the smallest fractional allocation.
Research Toolkit: Essential Reagents and Methodologies
Table 3: Essential Research Reagents and Methodologies for ALA Metabolism Studies
| Reagent/Methodology | Function/Application | Key Considerations |
|---|---|---|
| Stable Isotope-Labeled ALA (e.g., [13C]-ULA) | Metabolic tracer for in vivo conversion studies | Enables precise quantification without radiation exposure; requires GC-IRMS instrumentation |
| GC-MS with FAME Derivatization | Analysis of fatty acid composition and isotope enrichment | High sensitivity and specificity; enables simultaneous quantification of multiple PUFA |
| LC-MS-Based Oxylipin Profiling | Comprehensive analysis of PUFA-derived lipid mediators | Captures downstream functional metabolites; requires specialized sample preparation |
| FADS Genotyping Assays | Analysis of genetic variants in desaturase enzymes | Explains interindividual variability; requires DNA collection and genotyping platforms |
| Cell Culture Models (e.g., HepG2, C2C12) | In vitro investigation of metabolic pathways | Enables mechanistic studies; may not fully recapitulate in vivo metabolism |
| Red Blood Cell Fatty Acid Analysis | Biomarker of long-term PUFA status | Integrates exposure over erythrocyte lifespan (≈120 days); standardized protocols available |
Implications for Research and Clinical Practice
The consistently demonstrated inefficiency of ALA conversion to DHA, particularly in the context of modern Western diets high in LA, supports the classification of DHA as a conditionally essential nutrient [80] [6]. This has significant implications for dietary recommendations, particularly for populations with increased requirements such as pregnant women, infants, and individuals with specific genetic variants affecting FADS activity.
Future research directions should focus on:
- Development of precision nutrition approaches based on FADS genotyping
- Investigation of dietary and pharmacological strategies to enhance conversion efficiency (e.g., fucoxanthin supplementation shown to increase DHA conversion by 51.48% in quail models [85])
- Exploration of alternative pathways for increasing tissue DHA status without fish-derived sources
- Long-term studies on the health impacts of low conversion efficiency in aging populations
The biological constraints on DHA synthesis represent a significant consideration for pharmaceutical development, nutritional science, and public health policy, emphasizing the importance of direct DHA sources or alternative strategies to optimize omega-3 status in human populations.
Gender Differences in Metabolic Capacity and Implications
The investigation of gender differences in metabolic capacity represents a critical frontier in nutritional science, pharmacology, and clinical medicine. A comprehensive understanding of how biological sex influences nutrient processing, drug metabolism, and energy homeostasis has profound implications for personalized medicine and therapeutic development. This whitepaper delineates the fundamental physiological and hormonal mechanisms underpinning these dimorphisms, with particular emphasis on alpha-linolenic acid (ALA) metabolism as a model system. The emerging evidence confirms that gender-specific variations in metabolic pathways significantly impact disease susceptibility, drug efficacy, and nutritional requirements, necessitating gender-informed approaches in both research and clinical practice [86] [87].
The scope of this analysis extends from molecular pathways to whole-organism physiology, exploring how genetic, hormonal, and environmental factors interact to create distinct metabolic phenotypes in males and females. Within this framework, ALA metabolism serves as a paradigmatic example of how gender differences manifest in nutrient processing, with demonstrated variations in conversion efficiency to long-chain polyunsaturated fatty acids (LC-PUFA) between males and females [86]. This paper synthesizes current evidence from human studies, animal models, and clinical trials to provide researchers and drug development professionals with a comprehensive technical reference on gender-specific metabolic capacity.
Table 1: Key Quantitative Differences in Metabolic Parameters Between Genders
| Metabolic Parameter | Males | Females | Significance/Notes |
|---|---|---|---|
| Resting Metabolic Rate | 1,740 ± 194 kcal/day | 1,348 ± 125 kcal/day | 23% higher in males after controlling for body composition [88] |
| Fat-Free Mass | Higher | Lower (≈2/3 of males) | Accounts for majority of RMR difference [89] |
| Adipose Tissue | Lower | Higher (≈2× of males) | Different distribution patterns [89] |
| Skeletal Muscle Mass | Higher | Lower | Impacts glucose disposal and energy expenditure [89] |
| Hepatic DHA Synthesis | Lower | Higher | Females show 30-50% higher conversion of ALA to DHA [86] |
| Carbohydrate Oxidation | Higher | Lower | Males exhibit preferential carbohydrate metabolism [90] |
| Lipid Oxidation | Lower | Higher | Females exhibit preferential fat metabolism [90] |
| Drug Metabolism (CYP3A4) | Variable | Variable | Reversed dimorphism (F>M) in humans vs. rodents [91] |
Table 2: Gender Differences in Response to Nutritional Interventions
| Intervention | Male Response | Female Response | Clinical Implications |
|---|---|---|---|
| High-Carbohydrate Meal | Efficient glucose clearance | Enhanced glycogen storage | Women store more glycogen in liver and muscles [90] |
| High-Fat Meal | Delayed lipid clearance | Enhanced fatty acid utilization | Women show more effective postprandial fat metabolism [90] |
| Fasting | Rapid glycogen depletion | Preferential fat mobilization | Women release more free fatty acids during fasting [90] |
| ALA Supplementation | Lower EPA/DHA conversion | Higher EPA/DHA conversion | Hormonal regulation of desaturase enzymes [86] |
| Endurance Exercise | Greater glycogen utilization | Enhanced lipid oxidation | Women demonstrate better endurance in some contexts [92] |
Physiological Mechanisms Underlying Metabolic Dimorphisms
Hormonal Regulation of Metabolic Pathways
Sex hormones constitute the primary regulators of gender-specific metabolic patterns. Estradiol and progesterone positively correlate with increased LC-PUFA concentrations in plasma and tissues, while testosterone demonstrates a negative correlation with these parameters [86]. The mechanistic basis for these relationships involves hormonal regulation of hepatic desaturase and elongase enzymes responsible for the conversion of ALA to EPA and DHA. In females, the continuous pulsatile secretion pattern of growth hormone enhances expression of these enzymes, thereby facilitating more efficient LC-PUFA synthesis [91].
The impact of hormonal status extends to drug metabolism pathways, particularly cytochrome P450 (CYP) enzymes. Estrogen treatment has been demonstrated to inhibit CYP1A2 activity while inducing CYP3A4, CYP2B6, and CYP2D6-mediated metabolism [93]. These regulatory effects explain the clinically observed variations in drug clearance and side effect profiles between genders. For instance, women experience higher incidence of adverse drug reactions for medications metabolized by CYP3A4, which accounts for approximately 50% of all pharmaceutical biotransformation [87].
Body Composition and Energy Partitioning
Fundamental differences in body composition between genders significantly influence metabolic capacity. Males typically possess greater fat-free mass and skeletal muscle volume, accounting for their higher resting metabolic rate [88]. Conversely, females have proportionally more adipose tissue with distinct distribution patterns—subcutaneous deposition in gluteofemoral regions versus visceral accumulation in males [89]. This anatomical variation in fat storage has profound implications for metabolic health, as visceral adiposity strongly correlates with insulin resistance and cardiovascular risk.
The preferential fuel utilization patterns observed between genders—carbohydrate dominance in males versus lipid dominance in females—appear to be intrinsically linked to these body composition differences. Females demonstrate enhanced capacity for fatty acid uptake, intramuscular triglyceride synthesis, and lipid oxidation during exercise, attributable to higher activity of lipid-handling enzymes in skeletal muscle [92]. This metabolic flexibility provides an advantage during endurance activities but may complicate weight management strategies in sedentary conditions.
Alpha-Linolenic Acid Metabolism as a Paradigm of Gender Differences
Enzymatic Conversion and Hormonal Regulation
The metabolism of alpha-linolenic acid provides a compelling model for understanding gender-specific metabolic patterns. The conversion of ALA to longer-chain, more unsaturated derivatives including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) occurs via a series of elongation and desaturation reactions catalyzed by Δ-5 and Δ-6 desaturases [86]. Gender differences in the activity of these enzymes significantly impact the efficiency of this conversion process.
Table 3: Gender Differences in ALA Metabolism and Regulatory Factors
| Parameter | Males | Females | Regulatory Mechanism |
|---|---|---|---|
| Δ-6 Desaturase Activity | Lower | Higher | Estrogen-induced upregulation [86] |
| Hepatic EPA Synthesis | Reduced | Enhanced | Growth hormone secretion patterns [91] |
| Plasma DHA Concentrations | Lower | Higher | Positive correlation with estrogen [86] |
| Tissue LC-PUFA Accumulation | Diminished | Enhanced | Progesterone synergistic effects [86] |
| Response to Oral Contraceptives | N/A | Altered enzyme expression | Exogenous hormone effects [86] |
Experimental evidence indicates that female rats exhibit 30-50% higher conversion rates of ALA to DHA compared to males, paralleling observations in human subjects [86]. This enhanced conversion efficiency in females is positively associated with circulating concentrations of estradiol and progesterone, while inversely correlated with testosterone levels. The administration of oral contraceptives or hormone replacement therapy further modifies these metabolic pathways, confirming the central role of sex hormones in regulating ALA metabolism.
Implications for Health and Disease
The gender divergence in ALA metabolism has significant implications for health outcomes and disease risk profiles. The higher circulating and tissue concentrations of EPA and DHA in females may confer protective benefits against inflammatory and cardiovascular conditions, potentially contributing to the longer life expectancy observed in women. These LC-PUFAs serve as precursors for specialized pro-resolving mediators that actively promote resolution of inflammation, with demonstrated cardioprotective and neuroprotective effects [86].
From a therapeutic perspective, these metabolic differences necessitate gender-specific dosing strategies for ALA supplementation and LC-PUFA formulations. Drug development programs targeting the enzymes involved in ALA metabolism must account for gender-specific expression and activity patterns, particularly for conditions with unequal gender prevalence such as autoimmune disorders (female-predominant) and cardiovascular disease (male-predominant in younger cohorts).
Experimental Methodologies for Investigating Gender Differences
Human Studies and Clinical Trial Design
The gold standard for investigating gender differences in metabolic capacity involves randomized controlled trials with careful gender stratification. The preferred methodology includes stable isotope tracer techniques to quantify nutrient kinetics in vivo. For ALA metabolism studies, deuterated or carbon-13 labeled ALA is administered orally or intravenously, with subsequent tracking of conversion to EPA and DHA in plasma phospholipids over time [86].
Key considerations for clinical trial design include controlling for hormonal status in female participants (menstrual cycle phase, menopausal status, contraceptive use), as these factors significantly impact metabolic outcomes. Additionally, body composition assessment via DEXA scanning provides essential covariate data for interpreting gender differences in energy expenditure and substrate utilization [88]. Pharmacokinetic studies require sufficient recruitment of both genders to detect potentially clinically significant differences in drug metabolism and clearance [87].
Animal Models and In Vitro Systems
Rodent models, particularly rats, have been extensively utilized to investigate gender differences in metabolism due to the ability to impose strict dietary control and perform invasive tissue sampling [86]. The rat model demonstrates analogous gender differences in n-3 PUFA status to humans, with females exhibiting higher plasma DHA concentrations. Hormonal manipulation studies in rodents, including gonadectomy and hormone replacement, have elucidated the specific roles of estrogen, progesterone, and testosterone in regulating metabolic pathways [86].
In vitro approaches employing primary hepatocytes or hepatoma cell lines enable mechanistic studies of hormonal regulation at the cellular level. Experimental protocols typically involve treatment with physiological concentrations of sex hormones (estradiol: 1-10 nM, testosterone: 10-30 nM) followed by assessment of enzyme activity, gene expression, and metabolite production [93]. For ALA metabolism studies, radiolabeled or stable isotope-labeled substrates allow precise quantification of conversion efficiency to downstream products.
The Scientist's Toolkit: Essential Research Reagents and Methodologies
Table 4: Key Research Reagents and Methodologies for Investigating Gender Differences in Metabolism
| Reagent/Methodology | Application | Technical Function | Gender Research Utility |
|---|---|---|---|
| Stable Isotope Tracers (¹³C-ALA, ²H-ALA) | ALA kinetics and conversion efficiency | Metabolic pathway tracing | Quantifies gender differences in LC-PUFA synthesis [86] |
| Hormone Receptor Modulators (selective ER/AR agonists/antagonists) | Mechanistic studies | Hormonal pathway dissection | Elucidates sex hormone effects on metabolism [86] |
| GC-MS/MS and LC-MS/MS | Metabolite profiling | Comprehensive metabolite quantification | Identifies gender-specific metabolic patterns [94] |
| Gene Expression Arrays | Transcriptome analysis | Pathway-specific gene expression | Reveals hormonal regulation of metabolic genes [94] |
| Primary Hepatocyte Cultures | In vitro modeling | Liver metabolism studies | Controls hormonal milieu to isolate effects [93] |
| CYP Isoform-Specific Substrates | Drug metabolism phenotyping | Enzyme activity assessment | Characterizes gender differences in drug metabolism [87] |
| HS-SPME-GC-MS | Volatile metabolite analysis | Untargeted metabolomics | Discovers gender-specific volatile metabolites [94] |
Implications for Drug Development and Therapeutic Approaches
Pharmacokinetic and Pharmacodynamic Considerations
Gender differences in metabolic capacity have profound implications for drug development and clinical pharmacology. Significant variations in drug absorption, distribution, metabolism, and excretion (ADME) between men and women have been documented for numerous therapeutic agents [87]. These differences stem from variations in body composition, gastric emptying, plasma protein binding, and, most significantly, activity of drug-metabolizing enzymes.
The cytochrome P450 superfamily demonstrates particularly notable gender dimorphisms. CYP3A4, responsible for metabolizing over 50% of pharmaceutical agents, shows higher activity in women compared to men, leading to more rapid clearance of substrates including benzodiazepines, calcium channel blockers, and many antipsychotics [87]. Conversely, CYP1A2 activity is generally higher in men, resulting in gender-specific dosing requirements for substrates such as caffeine, clozapine, and theophylline. These metabolic differences contribute to the higher incidence of adverse drug reactions observed in women, underscoring the necessity of gender-informed prescribing practices [87].
Gender-Affirming Hormone Therapy and Metabolic Consequences
The administration of gender-affirming hormone therapy to transgender individuals provides a unique opportunity to study the metabolic effects of sex hormones in adults. Testosterone treatment in transgender men produces significant increases in lean body mass, resting metabolic rate, and insulin resistance, while estrogen therapy in transgender women promotes fat accumulation and insulin sensitivity [93]. These changes mirror the innate metabolic differences between cisgender men and women, confirming the central role of sex hormones in metabolic regulation.
From a clinical perspective, hormone therapy significantly impacts the pharmacokinetics of concomitant medications. Estrogen-based regimens have been demonstrated to induce CYP2B6, CYP2D6, and CYP3A4 activities, potentially necessitating dose adjustments for medications metabolized through these pathways [93]. Additionally, testosterone and estrogen therapies influence transporter protein expression, further modifying drug disposition patterns. These findings highlight the importance of considering hormonal status when prescribing medications to transgender individuals and suggest that hormone therapy may represent a useful experimental model for investigating hormonal regulation of metabolic pathways.
The evidence comprehensively detailed in this whitepaper confirms that gender represents a fundamental biological variable significantly impacting metabolic capacity across multiple physiological domains. From nutrient partitioning to drug biotransformation, the metabolic divergence between males and females demonstrates the pervasive influence of sex chromosomes and hormonal milieus on biochemical pathways. The metabolism of alpha-linolenic acid serves as an instructive model system, illustrating how gender differences manifest at the enzymatic level and translate to clinically significant variations in LC-PUFA status.
For researchers and drug development professionals, these findings underscore the critical importance of gender-informed approaches in both basic science and clinical applications. Future research directions should include expanded investigation of the molecular mechanisms linking sex hormones to metabolic regulation, development of gender-specific computational models predicting nutrient and drug disposition, and clinical trials explicitly designed to detect gender-based differential responses to nutritional and pharmaceutical interventions. The integration of gender as a key variable in metabolic research will advance the frontier of personalized medicine and yield more effective, tailored therapeutic strategies for both men and women.
Dose-Response Relationships and Therapeutic Window
This technical guide provides a comprehensive examination of dose-response relationships and therapeutic windows within the context of alpha-linolenic acid (ALA) research. We synthesize current evidence on ALA dosing, efficacy thresholds, and safety parameters to establish a framework for clinical translation. The analysis includes quantitative data synthesis, experimental protocol details, and visualization of key metabolic pathways to support researchers in nutraceutical and pharmaceutical development.
Alpha-linolenic acid (ALA) represents an essential omega-3 fatty acid with demonstrated cardiometabolic, neuroprotective, and anti-inflammatory properties. Understanding its dose-response characteristics is critical for establishing effective dosing strategies that maximize therapeutic benefits while minimizing potential risks. ALA, as a plant-derived essential fatty acid found predominantly in flaxseed, walnuts, and chia seeds, serves as a metabolic precursor to longer-chain omega-3 fatty acids EPA and DHA, though conversion rates are limited in humans [95] [96]. The therapeutic window for ALA supplementation depends on multiple factors including baseline health status, delivery matrix, and intervention duration, requiring sophisticated dose-response characterization to optimize clinical outcomes.
Quantitative Dose-Response Data Analysis
Table 1: Clinical Efficacy Outcomes of ALA Supplementation Across Dosing Regimens
| Health Outcome | Effective Dose Range | Intervention Duration | Key Efficacy Metrics | Population Characteristics |
|---|---|---|---|---|
| Cardiometabolic Risk Factors | 30-50 g/day whole flaxseed (≈6-10 g ALA) | ≥12 weeks | SBP reduction: 2-15 mmHg; DBP reduction: 1-7 mmHg; Lipid profile improvement [97] | High cardiometabolic risk individuals |
| Lipid Profile Modulation | 1-4 g/day ALA | 6-12 months | Triglycerides: 14.7% reduction; HDL: 22.4% increase; TC: 1.7% reduction [98] | Simulated disease population with lipid imbalances |
| Cardiovascular Risk Reduction | 1.2-2 g/day dietary ALA | 6+ years | 20% risk reduction for fatal CHD; 59% lower first heart attack risk [48] | General population and those with existing heart disease |
| Migraine Symptoms (with L-carnitine) | 350 mg/day ALA + 500 mg L-carnitine | 12 weeks | Frequency: -2.96 days; Severity: -1.6 points; Duration: -4.9 hours [21] | Women with migraine without aura |
Table 2: Therapeutic Window and Safety Parameters for ALA
| Parameter | Substrate/Matrix | Therapeutic Range | Safety Considerations | Risk Populations |
|---|---|---|---|---|
| Optimal Cardioprotection | Whole ground flaxseed | ≥30 g/day (≥12 weeks) | Well-tolerated; high-calorie content may promote weight gain [97] [48] | Individuals with obesity requiring calorie restriction |
| Prostate Cancer Risk | Animal-derived ALA | Dose-dependent risk | Increased risk with high intake from meat/dairy sources [48] | Men with personal/family history of prostate cancer |
| Prostate Cancer Risk | Plant-derived ALA | No significant risk | No association with prostate cancer risk [48] | General population |
| Triglyceride Levels | ALA supplements | Contraindicated | May worsen high triglyceride conditions [48] | Patients with hypertriglyceridemia |
Experimental Protocols for Dose-Response Characterization
Clinical Trial Design Considerations
Establishing ALA dose-response relationships requires carefully controlled trial methodologies. Adaptive trial designs provide flexibility to address uncertainties during planning and execution, increasing the chances of identifying optimal doses and improving trial efficiency [99]. Model-based methods, such as Emax modeling or MCP-Mod (which combines multiple comparison procedures and modeling), incorporate assumptions about the dose-response relationship to improve the precision of dose-response and target dose estimation [99]. For ALA interventions, trial design must account for its food matrix effects, with whole flaxseed demonstrating different bioavailability compared to refined oils.
Key design parameters:
- Dose selection: Include subclinical, clinical, and suprclinical doses if safe
- Duration: Minimum 12 weeks to assess cardiometabolic parameters
- Population stratification: Based on baseline risk factors and lipid profiles
- Endpoint selection: Combined clinical and biomarker endpoints
Systems Biology Modeling Approach
Recent advances employ in-silico modeling to predict ALA dose-response relationships. The systems biology-based mathematical modelling framework integrates pharmacokinetic and pharmacodynamic parameters to simulate lipid metabolism effects [98].
Protocol implementation:
- Pharmacokinetic model benchmarking: Fit two-compartment model with Michaelis-Menten and linear elimination using clinical PK data
- Pharmacodynamic liver model: Parameterize to reproduce cardiovascular conditions mimicking unhealthy lipid levels
- Dose-response simulation: Generate individual PK profiles for ALA and integrate into liver model
- Validation: Iteratively adjust parameters to match clinical outcomes from multiple trials
- Subgroup analysis: Identify populations with differential response based on baseline characteristics
This approach enables prediction of lipid profile changes (triglycerides, total cholesterol, LDL, HDL) across different ALA dosing regimens and identification of patient subgroups most likely to benefit from intervention [98].
Metabolic Pathways and Experimental Workflows
ALA Metabolism and Signaling Pathways
Diagram Title: ALA Metabolic Conversion and Therapeutic Mechanisms
Dose-Response Experimental Workflow
Diagram Title: Dose-Response Characterization Workflow
Research Reagent Solutions
Table 3: Essential Research Materials for ALA Dose-Response Studies
| Reagent/Resource | Function/Application | Specification Considerations |
|---|---|---|
| Flaxseed Oil Standardized Extracts | Intervention material for clinical trials | ALA concentration ≥50%; certified for purity and stability [21] |
| Gas Chromatography-Mass Spectrometry | ALA biomarker quantification in plasma/serum | Validated method for fatty acid profiling with appropriate internal standards [96] |
| Systems Biology Modeling Platform | In-silico prediction of dose-response relationships | Capable of PK/PD modeling and population subgroup analysis [98] |
| Placebo Matching Formulations | Control for clinical trials | Identical appearance/sensory properties without active ALA (e.g., paraffin oil) [21] |
| Lipid Profile Assay Kits | Assessment of cardiometabolic efficacy endpoints | Standardized measurement of triglycerides, HDL, LDL, total cholesterol [97] [98] |
| Inflammatory Marker Panels | Quantification of anti-inflammatory effects | Multiplex assays for CRP, IL-6, TNF-α [97] |
Dose-response characterization for ALA reveals a favorable therapeutic window for cardiometabolic and neuroprotective applications, with optimal efficacy at doses of 1.2-2 g/day for cardiovascular risk reduction and higher doses (30-50 g/day flaxseed) required for broader cardiometabolic benefits. The safety profile is generally excellent for plant-derived ALA, though precautions are warranted in specific risk populations. Future research should focus on precision nutrition approaches to identify patient subgroups with enhanced responsiveness, improved delivery systems to enhance bioavailability, and combination therapies that leverage synergistic nutrient interactions. Standardization of ALA dosing protocols and validation of biomarker-driven dosing strategies will advance the field toward personalized ALA interventions optimized through robust dose-response relationships.
Competition with Linoleic Acid in Metabolic Pathways
The metabolic pathways of alpha-linolenic acid (ALA, 18:3 n-3) and linoleic acid (LA, 18:2 n-6) represent a critical intersection in human lipid metabolism with profound implications for health and disease. As essential polyunsaturated fatty acids (PUFAs), both must be obtained through dietary sources, yet they compete vigorously for the same enzymatic machinery within biological systems [28]. This competition is not merely a biochemical curiosity but a fundamental regulatory mechanism that influences inflammatory responses, cellular membrane composition, and ultimately, cardiometabolic health outcomes.
The enzymatic preference observed in this competition has significant physiological consequences. ALA serves as the precursor for the omega-3 series of eicosanoids, which generally exhibit anti-inflammatory properties, while LA gives rise to the omega-6 series, which often promotes pro-inflammatory signaling [28]. The balance between these competing pathways therefore modulates the overall inflammatory tone within tissues and organs. Understanding the precise mechanisms governing this metabolic competition provides crucial insights for nutritional recommendations and therapeutic interventions targeting chronic inflammatory diseases, cardiovascular conditions, and metabolic disorders.
Biochemical Pathways and Enzymatic Mechanisms
Shared Enzymatic Machinery
The metabolic competition between ALA and LA unfolds primarily within the endoplasmic reticulum, where both substrates vie for the attention of a limited set of desaturase and elongase enzymes. The transformation of both fatty acids follows a similar pattern of alternating desaturation and elongation steps, yet with markedly different efficiencies and affinities [17].
Delta-6 desaturase (D6D) represents the initial rate-limiting step for both pathways and demonstrates a notable preference for ALA over LA. This enzymatic preference was clearly demonstrated in fetal rat studies, where delta-6 desaturation rates were significantly higher for alpha-linolenic acid than for linoleic acid [100]. Following desaturation, both pathways utilize elongase enzymes to add two-carbon units, followed by delta-5 desaturase for further desaturation. The final step in the production of docosahexaenoic acid (DHA) from ALA requires translocation to peroxisomes for partial beta-oxidation, adding another layer of complexity to the metabolic journey [17].
Tissue-Specific Metabolism
The competition between these fatty acids exhibits significant tissue variation, with certain organs demonstrating remarkable metabolic specialization. Research comparing hepatic and cerebral metabolism reveals that fetal brain tissue efficiently converts both linoleic and alpha-linolenic acid to long-chain derivatives, with desaturation rates actually exceeding those observed in liver tissue [100]. In the liver, however, alpha-linolenic acid is rapidly converted to long-chain derivatives in vivo, while linoleic acid metabolism appears more constrained [100]. This tissue-specific patterning suggests that different organs may employ distinct regulatory strategies to ensure adequate production of long-chain PUFAs despite the ongoing substrate competition.
Quantitative Analysis of Metabolic Competition
Conversion Efficiencies and Affinities
The metabolic competition between ALA and LA can be quantitatively assessed through conversion efficiencies, enzyme affinities, and the resulting impact on downstream products. The following table summarizes key quantitative differences in their metabolic handling:
Table 1: Quantitative Comparison of ALA and LA Metabolism
| Parameter | Alpha-Linolenic Acid (ALA) | Linoleic Acid (LA) |
|---|---|---|
| Delta-6 Desaturase Affinity | Higher affinity and conversion rate [100] | Lower affinity compared to ALA [100] |
| Hepatic Conversion to LCPUFAs | Rapid conversion in vivo [100] | Limited conversion under normal conditions [100] |
| Brain Conversion Efficiency | Efficiently converted to long-chain derivatives [100] | Efficiently converted to long-chain derivatives [100] |
| Typical Conversion to EPA | Approximately 0.3-8% in humans [28] | Not applicable |
| Typical Conversion to DHA | <0.1-4% in humans [28] | Not applicable |
| Impact of High LA Intake | Conversion to EPA/DHA significantly inhibited [28] | Enhanced conversion to AA and other n-6 LCPUFAs |
Impact of Dietary Ratios
The dietary ratio of LA to ALA exerts a profound influence on the outcomes of this metabolic competition. Modern Western diets typically exhibit an n-6/n-3 PUFA ratio of approximately 10:1, a significant departure from the evolutionary balanced ratio of 1:4-5 that supports optimal physiological function [28]. This imbalance creates a competitive disadvantage for ALA metabolism, as the excessive dietary LA saturates the shared enzymatic machinery and effectively reduces the production of EPA and DHA from ALA.
The consequences of this imbalance extend throughout the physiological system. High LA intake promotes the production of arachidonic acid (AA), which serves as the precursor for pro-inflammatory eicosanoids including series-2 prostaglandins and series-4 leukotrienes [28]. Conversely, adequate ALA intake and efficient conversion to EPA leads to the production of less inflammatory eicosanoids (series-3 prostaglandins and series-5 leukotrienes) and specialized pro-resolving mediators that actively promote inflammation resolution [28]. The competition at the enzymatic level therefore propagates upward to influence systemic inflammatory status and disease risk.
Experimental Approaches and Methodologies
Key Experimental Models
Research elucidating the competition between ALA and LA has employed diverse experimental models, each offering unique insights into different aspects of this metabolic relationship.
Table 2: Experimental Models for Studying ALA and LA Competition
| Model System | Application | Key Findings |
|---|---|---|
| In vivo fetal rat studies | Comparing metabolism of [1-¹â´C] linoleic acid vs. [1-¹â´C] alpha-linolenic acid | Demonstrated tissue-specific differences in conversion rates and higher delta-6 desaturation for ALA in brain tissue [100] |
| Human randomized controlled trials | Investigating effects of ALA supplementation on cardiovascular and metabolic parameters | ALA intake (2.13 g/d) improved brachial-ankle pulse wave velocity; increased serum n-3 fatty acids [101] |
| Epidemiological studies | Examining associations between ALA intake/biomarkers and chronic disease risk | Higher ALA levels associated with 10% lower risk of total CVD and 20% reduced risk of fatal CHD [95] |
| Cell culture models (e.g., CaCo-2) | Studying desaturation and elongation mechanisms | Provided insights into enzyme kinetics and substrate preferences [17] |
Detailed Experimental Protocol: In Vivo Metabolic Tracing
To investigate the metabolic competition between ALA and LA in an experimental setting, the following protocol adapted from foundational studies can be employed:
Objectives: To quantitatively compare the metabolism of radiolabeled ALA and LA in target tissues, including conversion rates to long-chain derivatives and tissue-specific distribution patterns.
Materials and Reagents:
- [1-¹â´C] linoleic acid (18:2 n-6) and [1-¹â´C] alpha-linolenic acid (18:3 n-3)
- Animal model (e.g., rat model, preferably including fetal developmental stages)
- Lipid extraction solvents: chloroform, methanol in 2:1 ratio (v/v)
- Thin-layer chromatography (TLC) system for lipid separation
- Gas chromatography-mass spectrometry (GC-MS) for fatty acid analysis
- Scintillation counter for radioactivity measurement
- Tissue homogenization equipment
Methodology:
- Administration: Administer equimolar quantities of [1-¹â´C]ALA and [1-¹â´C]LA via appropriate route (oral gavage or intravenous injection).
- Tissue Collection: After predetermined time intervals (e.g., 1h, 6h, 24h), collect target tissues including liver, brain, adipose tissue, and plasma.
- Lipid Extraction: Homogenize tissues and extract total lipids using chloroform:methanol (2:1) following the Folch method.
- Fractionation: Separate lipid classes by thin-layer chromatography using appropriate solvent systems.
- Analysis: Hydrolyze phospholipid and triglyceride fractions, derivative fatty acids, and analyze by GC-MS to identify and quantify metabolites.
- Quantification: Measure radioactivity in each metabolite fraction using scintillation counting.
- Data Analysis: Calculate conversion efficiencies as percentage of administered radioactivity appearing in each metabolite pool for both ALA and LA pathways.
This protocol enables direct comparison of the metabolic fates of these competing essential fatty acids and reveals tissue-specific differences in their handling, as demonstrated in the fetal rat model where brain tissue showed efficient conversion of both substrates while liver exhibited preferential metabolism of ALA [100].
Visualization of Metabolic Pathways
The following diagram illustrates the competitive metabolic pathways of ALA and LA, highlighting shared enzymatic machinery and key differences in their transformation to long-chain polyunsaturated fatty acids.
Diagram 1: Competitive Metabolic Pathways of ALA and LA
This visualization illustrates how ALA (green) and LA (red) compete for the same desaturase and elongase enzymes (gray) in their transformation to long-chain polyunsaturated fatty acids. The thicker arrows for ALA at the delta-6 desaturase step represent its higher affinity for this rate-limiting enzyme, as observed in experimental models [100]. The ALA pathway requires an additional peroxisomal beta-oxidation step for DHA production, while the LA pathway yields arachidonic acid as a key inflammatory precursor.
Research Reagents and Methodological Tools
Table 3: Essential Research Reagents for Studying ALA/LA Competition
| Reagent/Tool | Application | Specific Function |
|---|---|---|
| [1-¹â´C] ALA and [1-¹â´C] LA | Metabolic tracing studies | Radiolabeled substrates to quantitatively track conversion to elongated/desaturated products and compare metabolic rates [100] |
| GC-MS systems | Fatty acid analysis | Precise identification and quantification of fatty acid metabolites in tissues and biological fluids |
| Delta-6 desaturase inhibitors | Enzyme activity studies | Pharmacological tools to investigate consequences of disrupting the competitive balance between substrates |
| ALA-rich oils (flaxseed, perilla, chia) | Human intervention studies | Dietary sources to examine effects of increased ALA intake on fatty acid profiles and health outcomes [28] |
| LA-rich oils (sunflower, corn, soybean) | Competition studies | Used to create high LA conditions that inhibit ALA conversion to examine metabolic consequences [28] |
| Cell culture models (CaCo-2, hepatocytes) | In vitro mechanism studies | Controlled systems for investigating enzyme kinetics, substrate preferences, and regulatory mechanisms [17] |
Health Implications and Research Applications
Cardiovascular and Metabolic Health
The competition between ALA and LA has significant implications for cardiovascular disease risk, with substantial evidence supporting the cardioprotective effects of ALA. Meta-analyses of observational studies have demonstrated that increasing dietary ALA is associated with a 10% lower risk of total cardiovascular disease and a 20% reduced risk of fatal coronary heart disease [95]. Randomized controlled trials have further elucidated that ALA intake contributes to cardiovascular risk reduction through multiple mechanisms, including improvements in lipid profiles (reducing total cholesterol, LDL cholesterol, and triglycerides), blood pressure reduction, and enhanced vascular function as measured by brachial-ankle pulse wave velocity [101] [95].
The relationship between ALA and metabolic syndrome components reveals a complex interplay. While evidence remains somewhat inconsistent, several studies indicate favorable effects of ALA on abdominal obesity, triglyceride levels, and hypertension [28]. The unique physiological effects of ALA during weight loss were demonstrated in a 6-month hypoenergetic diet trial where supplementation with ALA-rich rapeseed oil induced significantly greater reductions in diastolic blood pressure and triglyceride levels compared to an oleic acid-rich diet [28]. These metabolic benefits appear to derive not only from ALA's conversion to EPA but also from its direct effects on oxylipin profiles and endocannabinoid signaling [28].
Inflammation and Oxylipin Production
The competitive relationship between ALA and LA fundamentally influences inflammatory processes through their respective roles as precursors to distinct oxylipin families. ALA serves as the precursor to the omega-3 series of oxylipins, which generally exhibit anti-inflammatory and pro-resolving properties, while LA gives rise to the omega-6 series, which includes many pro-inflammatory mediators [28]. The balance between these competing pathways therefore establishes the inflammatory tone at the tissue level.
Research indicates that ALA exerts systemic anti-inflammatory effects, particularly in populations with elevated inflammatory risk such as individuals with overweight/obesity and metabolic syndrome [28]. These effects are mediated through multiple mechanisms, including modulation of the n-3/n-6 PUFA ratio in cell membranes, subsequent alteration of eicosanoid production patterns, and influence on endocannabinoid signaling pathways [28]. The direct impact of ALA on oxylipin profiles represents an active area of investigation, with recent studies employing lipidomic approaches to characterize the specific oxylipins generated from ALA and their biological activities.
Research Gaps and Future Directions
Despite significant advances in understanding ALA and LA competition, important research questions remain unresolved. The conversion efficiency of ALA to EPA and DHA shows considerable interindividual variation influenced by genetic polymorphisms, hormonal status, and dietary context, yet personalized approaches to optimizing this conversion remain underdeveloped [28] [95]. The specific mechanisms through which ALA exerts direct physiological effects independent of its conversion to long-chain PUFAs represent another area requiring further investigation [28].
Future research directions should include:
- Long-term intervention studies specifically designed to examine how varying ALA:LA ratios influence hard clinical endpoints
- Integration of omics technologies (lipidomics, genomics, transcriptomics) to identify biomarkers of metabolic efficiency and personalized optimal ratios
- Investigation of ALA's role in cognitive health and neuroprotection, an emerging area with promising preliminary evidence [95]
- Development of nutritional and pharmacological strategies to enhance ALA conversion to EPA and DHA, particularly in populations with inefficient metabolism
Understanding the competitive dynamics between these essential fatty acids continues to provide important insights for developing targeted nutritional strategies to combat chronic inflammatory diseases, cardiovascular disorders, and metabolic conditions.
Strategies to Enhance ALA Bioefficacy
Alpha-linolenic acid (ALA), an essential omega-3 polyunsaturated fatty acid, demonstrates significant cardiometabolic benefits and potential to promote healthy aging. However, its therapeutic application is often constrained by challenges related to its bioavailability and stability. This technical guide synthesizes current scientific strategies to enhance ALA bioefficacy, providing researchers and drug development professionals with methodologically rigorous approaches to overcome these limitations. We present a comprehensive analysis of formulation technologies, chemical modifications, and delivery systems designed to optimize ALA's pharmacokinetic profile and therapeutic potential, framed within the context of advanced ALA metabolism research.
Alpha-linolenic acid (ALA) serves as a crucial precursor to longer-chain omega-3 fatty acids and exhibits direct biological effects, including anti-inflammatory properties, antioxidant activity, and cardiometabolic risk reduction [97]. Despite these promising characteristics, ALA's efficacy is compromised by several inherent limitations:
- Low aqueous solubility: ALA’s hydrophobic nature restricts its dissolution in gastrointestinal fluids, reducing absorption potential.
- Chemical instability: The unsaturated bonds in ALA are susceptible to oxidative degradation, compromising potency and shelf life.
- Hepatic first-pass metabolism: Significant pre-systemic metabolism limits bioavailability following oral administration.
- Variable absorption: Bioavailability is influenced by dietary matrix, genetic factors, and individual metabolic differences.
These challenges necessitate strategic interventions to enhance ALA's delivery and therapeutic performance, particularly for clinical applications targeting cardiometabolic diseases and age-related health deterioration [97].
Formulation-Based Enhancement Strategies
Lipid-Based Delivery Systems
Lipid-based formulations represent a prominent approach to improve ALA bioavailability by enhancing solubility and promoting lymphatic absorption, which bypasses first-pass metabolism.
Table 1: Lipid-Based Delivery Systems for ALA Bioavailability Enhancement
| System Type | Composition | Mechanism of Action | Reported Efficacy |
|---|---|---|---|
| Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) | ALA, surfactants, co-surfactants, oil phase | Forms fine oil-in-water nanoemulsions in GI tract; enhances solubility and permeability | >2-fold increase in bioavailability compared to conventional formulations [102] |
| Nanoemulsions | ALA, emulsifiers, aqueous phase | Submicron droplet size increases surface area for absorption; improves intestinal permeability | Enhanced stability and penetration through biological barriers [103] |
| Solid Lipid Nanoparticles (SLNPs) | Lipid core stabilized by surfactants | Protects ALA from degradation; controls release kinetics | Improved cellular uptake and prolonged circulation time [102] |
Experimental Protocol: SNEDDS Formulation Development
- Component Selection: Combine ALA (active ingredient) with medium-chain triglycerides (oil phase), Tween 80 (surfactant), and PEG-400 (co-surfactant) in optimized ratios.
- Pseudoternary Phase Diagram Construction: Systematically vary oil:surfactant:co-surfactant ratios to identify nanoemulsion region boundaries.
- Emulsification Efficiency Assessment: Dilute optimal SNEDDS formulation with simulated intestinal fluid (1:100 v/v) under gentle agitation.
- Characterization: Evaluate droplet size (<100 nm target) by dynamic light scattering, polydispersity index (<0.3), and emulsification time (<2 minutes).
- In Vitro Release Testing: Utilize dialysis membrane method in simulated gastrointestinal fluids with sink conditions; analyze ALA content via HPLC.
- Stability Studies: Monitor physical and chemical stability under accelerated conditions (40°C ± 2°C/75% RH ± 5% RH) for 6 months [102].
Solid Dispersion Technologies
Solid dispersions incorporate ALA into hydrophilic polymer matrices to create amorphous systems with enhanced dissolution properties.
Table 2: Polymer Carriers for ALA Solid Dispersions
| Polymer System | Properties | Commercial Precedents | Enhancement Mechanism |
|---|---|---|---|
| Hydroxypropyl Methylcellulose (HPMC) | Hydrophilic polymer; swellable matrix | Nivadil (Nilvadipine), PROGRAF (Tacrolimus) [102] | Inhibits crystallization; maintains supersaturation |
| Polyvinylpyrrolidone (PVP) | Water-soluble polymer; strong hydrogen bond acceptor | Cesamet (Nabilone), REZULIN (Troglitazone) [102] | Molecular dispersion; improved wetting |
| Hydroxypropyl Methylcellulose Acetate Succinate (HPMCAS) | pH-dependent solubility | INCIVEK (Telaprevir) [102] | Precipitates in gastric pH; dissolves in intestinal pH |
Experimental Protocol: Hot Melt Extrusion for Solid Dispersions
- Physical Mixture Preparation: Blend ALA with polymer carrier (e.g., HPMC, PVP-VA) in optimized ratios (typically 1:1 to 1:4 drug:polymer).
- Extrusion Parameters: Utilize twin-screw hot melt extruder with temperature profile calibrated below ALA's decomposition point but above polymer glass transition.
- Process Monitoring: Record torque, screw speed (50-200 rpm), and temperature to ensure homogeneous dispersion.
- Product Characterization: Assess amorphous nature by X-ray diffraction, homogeneity by DSC, and dissolution profile in biorelevant media.
- Storage Stability: Package in foil bags with desiccant and monitor crystallization tendency under ICH stability guidelines [102].
Chemical Modification Approaches
Prodrug Strategies
Prodrug development modifies ALA's chemical structure to overcome physicochemical limitations, with enzymatic conversion releasing active ALA at the target site.
Figure 1: ALA Prodrug Bioactivation Pathway - Chemical modifications enhance cellular uptake before enzymatic conversion to active ALA
Experimental Protocol: ALA Prodrug Synthesis and Evaluation
- Esterification Reaction: React ALA with appropriate alcohol (methyl, ethyl, hexyl) using dicyclohexylcarbodiimide (DCC) as coupling agent and 4-dimethylaminopyridine (DMAP) as catalyst in anhydrous dichloromethane.
- Reaction Monitoring: Track progress by thin-layer chromatography (silica gel, petroleum ether:ethyl acetate 4:1 as mobile phase).
- Purification: Isolate product via flash column chromatography; characterize by NMR, mass spectrometry, and HPLC for purity assessment.
- Stability Studies: Evaluate chemical stability in simulated gastric and intestinal fluids (pH 1.2 and 6.8, respectively).
- Enzymatic Conversion Assessment: Incubate with esterases (porcine liver esterase, 37°C) and monitor ALA release kinetics.
- Cellular Uptake Studies: Utilize Caco-2 cell monolayers to measure apparent permeability coefficients; compare with unmodified ALA [103].
Nanoparticulate Systems
Nanoparticle technology encapsulates ALA to protect it from degradation and enhance targeted delivery.
Experimental Protocol: ALA-Loaded Nanoparticle Preparation
- Nanoprecipitation Method:
- Dissolve ALA and polymer (PLGA, PLA) in water-miscible organic solvent (acetone, ethanol).
- Inject organic solution into aqueous phase containing stabilizer (poloxamer, polysorbate 80) under magnetic stirring.
- Evaporate organic solvent under reduced pressure; concentrate if necessary.
- Characterization Parameters:
- Particle size and zeta potential: Dynamic light scattering
- Entrapment efficiency: Ultracentrifugation followed by HPLC analysis
- Morphology: Transmission electron microscopy
- In vitro release: Dialysis method in biorelevant media
- Cell Culture Evaluation:
- Cytotoxicity assessment (MTT assay) in relevant cell lines
- Cellular uptake studies with fluorescently labeled nanoparticles
- Antioxidant effect measurement (ROS scavenging assays) [102]
Metabolic Pathway Targeting Strategies
Enhancing ALA bioefficacy extends beyond delivery optimization to include modulation of its metabolic fate and biological activity.
Figure 2: ALA Metabolic Pathway and Enhancement Strategies - Comprehensive view of ALA metabolism from absorption to biological effects
Experimental Protocol: Evaluating ALA Metabolite Conversion
- Cell Culture Model:
- Utilize hepatocyte cell line (HepG2) or primary hepatocytes
- Treat with enhanced ALA formulations (0.1-0.5 mM) for 24-72 hours
- Include FADS2 (delta-6-desaturase) activator/inhibitor controls
- Lipid Extraction and Analysis:
- Extract lipids using Folch method (chloroform:methanol 2:1 v/v)
- Derivatize fatty acids to methyl esters (FAMEs) using BF3-methanol
- Analyze by GC-MS with appropriate standards (ALA, EPA, DPA, DHA)
- Calculate conversion efficiency as (product)/(substrate + product) × 100
- Gene Expression Profiling:
- Isolate total RNA; synthesize cDNA
- Perform qRT-PCR for FADS1, FADS2, ELOVL2, ELOVL5 expression
- Normalize to housekeeping genes (GAPDH, β-actin) [97]
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Research Reagents for ALA Bioefficacy Studies
| Reagent/Category | Specific Examples | Research Application | Key Function |
|---|---|---|---|
| Specialized Polymers | HPMC, HPMCAS, PVP, PVP-VA, PEG [102] | Solid dispersion formulations | Maintain supersaturation; inhibit crystallization |
| Lipid Excipients | Medium-chain triglycerides, glycerol monooleate, oleic acid [102] [103] | Lipid-based delivery systems | Enhance solubility and lymphatic transport |
| Permeation Enhancers | 1-[2-(decylthio)-ethyl]azacyclopentan-2-one, 6-ketocholestanol [103] | Topical/formulation studies | Improve transmucosal and transdermal delivery |
| Analytical Standards | ALA (≥99%), EPA, DHA, deuterated internal standards | Bioanalysis and metabolite quantification | Accurate quantification in biological matrices |
| Esterase Enzymes | Porcine liver esterase, cholesterol esterase | Prodrug conversion studies | Evaluate enzymatic activation of ALA prodrugs |
| Cell Culture Models | Caco-2, HepG2, primary hepatocytes | Permeability and metabolism studies | Predict intestinal absorption and hepatic conversion |
Enhancing ALA bioefficacy requires a multidisciplinary approach integrating advanced formulation design, strategic chemical modification, and targeted metabolic interventions. The methodologies outlined in this technical guide provide a robust framework for researchers developing next-generation ALA-based therapeutics. Future directions should focus on personalized delivery systems accounting for genetic polymorphisms in fatty acid desaturases, combinatorial approaches that simultaneously enhance absorption and conversion efficiency, and clinical validation of these strategies in target populations with specific cardiometabolic conditions. As research continues to elucidate the complex relationship between ALA bioavailability and its systemic health effects, these enhancement strategies will play an increasingly vital role in maximizing ALA's therapeutic potential for promoting healthy aging and preventing chronic disease.
Individual Variability in Metabolic Response
The metabolism of alpha-linolenic acid (ALA), an essential omega-3 fatty acid, exhibits profound interindividual variability that significantly moderates its translational health benefits. This technical review examines the genetic, hormonal, and dietary factors governing differential metabolic responses to ALA, with particular focus on its conversion to long-chain polyunsaturated fatty acids (LC-PUFA). We synthesize current evidence on the implications of this metabolic heterogeneity for research design and therapeutic development, providing structured experimental protocols and analytical frameworks for investigating sources of variability in human populations.
Alpha-linolenic acid (ALA; 18:3n-3) serves as the essential plant-derived omega-3 fatty acid precursor for the synthesis of longer-chain, more unsaturated fatty acids, principally eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3) [6]. The fundamental biological importance of ALA lies in its role as substrate for these LC-PUFA, which are integral to cell membrane structure, neuronal function, and inflammatory regulation [104] [6]. Despite its essentiality, ALA metabolism demonstrates remarkable heterogeneity within human populations, creating significant challenges for predicting clinical outcomes and formulating targeted nutritional interventions.
This variability stems from a complex interplay between genetic polymorphisms, endocrine influences, dietary patterns, and competing metabolic pathways [30] [6] [105]. Understanding these sources of heterogeneity is paramount for researchers investigating ALA metabolism and pharmaceutical developers creating targeted metabolic therapies. This review provides a comprehensive examination of the factors underlying individual variability in ALA metabolic response, with specific methodological guidance for investigating and accounting for these differences in research and clinical applications.
ALA Metabolic Pathways and Conversion Efficiency
Biochemical Pathways of ALA Metabolism
Following ingestion, ALA undergoes several potential metabolic fates: incorporation into tissue lipid pools, β-oxidation for energy production, recycling of carbon into saturated and monounsaturated fatty acids, or conversion to LC-PUFA through a series of elongation and desaturation reactions [104]. The canonical pathway for LC-PUFA synthesis involves initial Δ6-desaturation to stearidonic acid (18:4n-3), elongation to eicosatetraenoic acid (20:4n-3), Δ5-desaturation to EPA, and subsequent elongation and desaturation through multiple steps to form DHA [17]. The final synthesis of DHA requires peroxisomal β-oxidation, adding another layer of potential metabolic regulation [17].
Diagram: Primary ALA Metabolic Pathways
Quantitative Conversion Efficiencies
The conversion of ALA to its longer-chain metabolites demonstrates significant gender-dependent variation, with consistently higher efficiency observed in women compared to men. The table below summarizes key quantitative findings from stable isotope tracer studies and dietary intervention trials.
Table 1: ALA to LC-PUFA Conversion Efficiencies by Gender
| Metabolic Parameter | Men | Women | Study Type | References |
|---|---|---|---|---|
| ALA to EPA Conversion | ~8% | ~21% | Stable Isotope Tracer | [30] [6] |
| ALA to DHA Conversion | 0-4% | ~9% | Stable Isotope Tracer | [30] [6] |
| Fractional Oxidation | 15-33% | ~22% | 13C-Labeled ALA | [104] |
| Carbon Recycling to SFA/MUFA | ~25% | Lower than men | 13C-Labeled ALA | [104] |
The limited conversion efficiency, particularly for DHA synthesis in men, suggests that EPA and DHA may be considered conditionally essential nutrients, necessitating direct dietary intake in certain populations [6].
Genetic Determinants
Genetic polymorphisms in fatty acid desaturase (FADS) genes account for approximately 30% of the variability in blood concentrations of omega-3 fatty acids among individuals [6]. Two common haplotypes in the FADS gene cluster (D and I) demonstrate dramatically different capacities for generating LC-PUFA. Haplotype D is associated with increased FADS1 and FADS2 activity, resulting in higher conversion rates of ALA to EPA and DHA [6]. These genetic variations substantially modify an individual's capacity to endogenously synthesize LC-PUFA from plant-derived precursors.
Table 2: Genetic Factors Influencing ALA Metabolic Variability
| Gene/Enzyme | Function in ALA Metabolism | Impact of Polymorphisms | Clinical Consequences | |
|---|---|---|---|---|
| FADS2 (Δ6-desaturase) | Initial desaturation of ALA to SDA | Altered catalytic efficiency | Up to 30% variability in LC-PUFA synthesis | [6] |
| FADS1 (Δ5-desaturase) | Desaturation of 20:4n-3 to EPA | Modified enzyme activity | Differences in EPA production capacity | [6] |
| Elongase Enzymes | Carbon chain elongation | Variable expression/activity | Altered EPA to DHA conversion efficiency | [105] |
| Peroxisomal Enzymes | Final DHA synthesis step | Transport/oxidation differences | Impaired DHA production independent of prior steps | [17] |
Hormonal and Gender Effects
Estrogen plays a significant regulatory role in ALA metabolism, partially explaining the pronounced gender differences in conversion efficiency. The higher endogenous estrogen levels in women of reproductive age upregulate Δ6-desaturase activity, thereby enhancing the conversion of ALA to downstream metabolites [30] [6]. This endocrine influence represents a crucial consideration for study design and population-specific dietary recommendations.
Dietary and Environmental Modulators
Several dietary factors significantly influence ALA metabolism and contribute to interindividual variability:
- Linoleic Acid Intake: As the primary omega-6 fatty acid, LA competes with ALA for the same desaturase and elongase enzymes, with most Western diets characterized by an n-6:n-3 ratio of approximately 10:1 compared to the recommended 1:4-5 [105]
- Dietary LC-PUFA Intake: High consumption of pre-formed EPA and DHA downregulates the expression and activity of Δ5- and Δ6-desaturases through feedback inhibition [6]
- Caloric Restriction and Weight Loss: Hypoenergetic diets, particularly those enriched with ALA sources like rapeseed oil, induce favorable changes in metabolic parameters including diastolic blood pressure and triglyceride levels [105]
- Macronutrient Composition: Dietary patterns affecting insulin sensitivity indirectly influence ALA metabolism through modifications in lipid trafficking and oxidative partitioning
Comorbidities and Metabolic Status
Metabolic syndrome (MetS) and its component conditions significantly alter ALA metabolism and utilization. The pro-inflammatory state characteristic of MetS, driven by adipose tissue hypoxia and macrophage infiltration, modifies the partitioning of ALA toward inflammatory mediator production rather than structural incorporation [105] [78]. Obesity-associated insulin resistance impairs the activity of desaturase enzymes, further reducing LC-PUFA synthesis from ALA precursors [78].
Diagram: Factors Governing Individual Variability in ALA Response
Experimental Approaches for Investigating Metabolic Variability
Stable Isotope Tracer Methodologies
Protocol: Controlled Tracer Administration for ALA Kinetics
- Tracer Preparation: Prepare [U-13C] ALA complexed with albumin or incorporated into a standardized meal (typical dose: 0.5-1 mg/kg body weight)
- Administration: Administer orally after overnight fast with a standard meal to ensure consistent absorption
- Sample Collection: Collect blood samples at baseline, 2, 4, 6, 8, 12, 24, 48, and 72 hours post-administration for plasma lipid analysis
- Breath Collection: Measure 13CO2 enrichment in breath samples to quantify β-oxidation
- Tissue Sampling: Adipose tissue biopsies at 24-72 hours for assessment of tracer incorporation
- Analytical Methods: GC-MS analysis of fatty acid methyl esters with isotopomer distribution
This approach enables precise quantification of ALA partitioning among oxidative, recycling, and conversion pathways while controlling for dietary background [104].
Dietary Intervention Studies
Protocol: Standardized ALA Supplementation Trial
- Study Population: Stratify participants by FADS genotype, gender, and metabolic health status
- Run-in Period: 2-week controlled diet with fixed n-6:n-3 ratio
- Intervention: Supplement with 10-15 g/day flaxseed oil (providing ~5-7.5 g ALA) or matched control
- Duration: Minimum 8 weeks to assess steady-state incorporation
- Outcome Measures:
- Erythrocyte membrane fatty acid composition
- Plasma phospholipid EPA and DHA levels
- Inflammatory biomarkers (CRP, IL-6, TNF-α)
- Oxylipin profiling by LC-MS
- Statistical Analysis: Multivariate regression to identify predictors of conversion efficiency
This design permits assessment of both metabolic conversion and functional outcomes while accounting for baseline variables [105].
Genotype-Stratified Analyses
Protocol: FADS Genotyping and Phenotype Correlation
- Genotyping: Identify rs174537 (FADS1), rs174576 (FADS2), and rs3834458 polymorphisms using TaqMan assays
- Phenotyping: Measure Δ5- and Δ6-desaturase activities as product:precursor ratios (ARA:DGLA and EPA:ALA)
- Stratification: Group participants by haplotype combination (DD, DI, II)
- Association Testing: Correlate genotype with:
- Fasting and postprandial LC-PUFA levels
- ALA conversion efficiency following standardized challenge
- Inflammatory mediator production
This approach directly quantifies the contribution of genetic variation to observed metabolic differences [6].
Research Reagent Solutions
Table 3: Essential Research Tools for Investigating ALA Metabolic Variability
| Reagent/Category | Specific Examples | Research Application | Key Considerations | |
|---|---|---|---|---|
| Stable Isotope Tracers | [U-13C] ALA, [d5] ALA | Kinetic studies of ALA metabolism | >96% absorption efficiency; enables precise metabolic fate tracing | [104] |
| Analytical Standards | Deuterated EPA, DHA, oxylipins | Quantitative MS-based analysis | Essential for accurate quantification of low-abundance metabolites | [105] |
| Genotyping Assays | FADS1/2 TaqMan SNP panels | Genetic stratification | Identifies ~30% of variability in omega-3 status | [6] |
| Specialized Diets | ALA-enriched, n-6 controlled | Dietary intervention studies | Controls for competing substrate effects | [105] |
| Oxylipin Panels | LC-MS/MS methods | Functional metabolic output | Measures physiologically active metabolites | [105] |
| Cell Models | Hepatocytes, enterocytes | Mechanistic studies | Enables controlled investigation of specific pathways | [17] |
Implications for Research and Therapeutic Development
Clinical Trial Design Considerations
The substantial individual variability in ALA metabolism necessitates sophisticated clinical trial designs that account for major sources of heterogeneity. Stratified randomization by FADS genotype and gender is essential for adequately powered investigations of ALA interventions. Adaptive trial designs that permit enrichment of responsive subpopulations may enhance detection of efficacy signals in early-phase clinical studies.
Secondary analyses should specifically examine genotype × treatment interactions and baseline n-6:n-3 ratio as effect modifiers. For trials targeting metabolic outcomes in MetS populations, careful characterization of insulin sensitivity and adipose tissue distribution provides critical covariates for interpreting response heterogeneity [105] [78].
Personalized Nutrition and Therapeutics
The recognition of profound interindividual differences in ALA metabolism supports a personalized approach to omega-3 nutritional recommendations and therapeutic development. Individuals carrying FADS haplotypes associated with reduced conversion efficiency (approximately 30% of the population) may derive substantially greater benefit from direct EPA/DHA supplementation rather than ALA precursors [6].
Future therapeutic strategies targeting the metabolic syndrome might leverage ALA responsiveness as a stratification biomarker, with ALA-responsive subpopulations potentially benefiting more from dietary interventions while non-responders might require more direct LC-PUFA supplementation or pharmacological approaches [105].
Individual variability in ALA metabolic response represents a fundamental consideration for research and therapeutic development. The complex interplay between genetic predisposition, endocrine status, dietary patterns, and metabolic health creates a heterogeneous landscape of ALA utilization and efficacy. Methodologically rigorous approaches that account for these sources of variation—through appropriate stratification, controlled dietary conditions, and comprehensive phenotyping—are essential for advancing our understanding of ALA metabolism and translating this knowledge into targeted, effective nutritional and therapeutic strategies.
Technical Challenges in Metabolic Flux Measurement
Metabolic flux analysis (MFA) represents a cornerstone technique in systems biology for quantifying the rates of metabolic reactions through biochemical pathways. In the specific context of alpha-linolenic acid (ALA) metabolism research, flux measurements face unique technical hurdles that can impact the validity and interpretation of health benefit studies. Fluxomics, defined as the measurement of all intracellular fluxes in central metabolism, integrates data from genomics, transcriptomics, proteomics, and metabolomics to provide a dynamic portrait of molecular interactions [106]. Understanding the flux through ALA metabolic pathways—particularly its conversion to longer-chain omega-3 fatty acids like EPA and DHA—is crucial for elucidating its controversial efficacy in improving health outcomes [50] [107]. This technical guide examines the core challenges, methodologies, and future directions in metabolic flux measurement, with specific application to ALA metabolism research.
Core Analytical Challenges in Flux Measurement
Limitations in Analytical Technologies
The measurement of metabolic fluxes relies primarily on two analytical platforms: nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS). Each technology presents distinct advantages and limitations for flux analysis, particularly when applied to complex metabolic pathways like ALA metabolism [106].
NMR spectroscopy offers several unique advantages for fluxomic studies, including non-destructive analysis, minimal sample preparation requirements, and the ability to recover samples for additional analyses. NMR provides structural elucidation capabilities and can monitor molecular movements, flexibility, reactions, and binding events across multiple time scales. Crucially, 1H-NMR is inherently quantitative, making it a powerful tool for metabolomics studies [106]. However, NMR suffers from two fundamental limitations: low sensitivity and spectral overlap. Despite significant improvements through dynamic nuclear polarization (DNP), cryo-probes, and ultra-high magnetic fields, NMR sensitivity remains insufficient for detecting many metabolites at physiological concentrations, particularly secondary metabolites and those involved in ALA metabolism that may exist at very low concentrations [106].
Mass spectrometry approaches, particularly when coupled with chromatography methods (GC-MS or LC-MS), provide significantly higher sensitivity compared to NMR. These techniques are capable of detecting volatile metabolites and those at very low concentrations beyond NMR's detection limits. The combination of MS with chromatographic separation helps overcome peak overlap issues encountered in NMR [106]. However, MS-based methods introduce their own challenges, including requiring extensive sample preparation, derivatization steps for non-volatile compounds, and destruction of samples during analysis. Additionally, MS does not readily provide the structural information or molecular interaction data that NMR offers [106].
Integration of Complementary Platforms
Given the complementary strengths and weaknesses of NMR and MS, integrating both platforms provides the most comprehensive approach for flux measurement in ALA metabolism studies. This combined approach leverages MS sensitivity for low-abundance metabolites while utilizing NMR for structural validation and quantitative analysis [106]. Such integration is particularly valuable when studying the complex conversion pathways of ALA to EPA and DHA, where multiple enzymatic steps occur at varying rates and intermediate metabolites exist at different concentrations [50].
Table 1: Comparison of Analytical Platforms for Metabolic Flux Analysis
| Parameter | NMR Spectroscopy | Mass Spectrometry |
|---|---|---|
| Sensitivity | Low (μM-mM range) | High (pM-nM range) |
| Quantitation | inherently quantitative | Requires internal standards |
| Structural Information | Excellent for molecular structure | Limited without standards |
| Sample Preparation | Minimal | Extensive (derivatization needed) |
| Sample Recovery | Possible | Destructive |
| Throughput | Moderate to high | Moderate |
| Metabolite Coverage | Limited by sensitivity and overlap | Broad with proper separation |
| Dynamic Range | Limited | Extensive |
Computational and Methodological Frameworks
Metabolic Flux Analysis (MFA) and Flux Balance Analysis (FBA)
Metabolic Flux Analysis represents a constraint-based approach for estimating intracellular fluxes within a defined metabolic network. MFA utilizes extracellular measurements including substrate uptake and product formation rates to determine carbon flux distribution [108]. In studies of xylose-fermenting yeasts, researchers successfully constructed stoichiometric models containing 39 reactions and 35 metabolites to simulate intracellular carbon flux, validating metabolome data with correlations above 90% for most metabolites [108].
Flux Balance Analysis (FBA) provides a mathematical framework for analyzing metabolite flow through metabolic networks. This approach relies on stoichiometric constraints representing the biochemical transformations in a metabolic network, with the stoichiometric matrix S containing stoichiometric coefficients for each reaction [109]. The mass balance equation at steady state is represented as:
Sv = 0
where v is the flux vector through all reactions. FBA uses linear programming to identify optimal flux distributions that maximize or minimize a specified objective function, typically biomass production or ATP synthesis [109]. For ALA metabolism studies, FBA can predict flux distributions through the various potential pathways of ALA utilization, including β-oxidation, carbon recycling, and conversion to longer-chain PUFAs [50].
Advanced Integration with Machine Learning and Kinetic Models
Recent advances in flux analysis involve integrating FBA with machine learning (ML) approaches to handle the growing complexity of genome-scale metabolic models and heterogeneous biological datasets [110]. ML algorithms serve as a bridge between knowledge-driven predictive FBA models and diverse experimental data, enabling both predictive and descriptive modeling. Supervised learning creates mappings between input data and defined output variables, while unsupervised learning identifies emergent patterns without predefined labels [110].
The integration of kinetic models with FBA addresses the limitation of steady-state assumptions in traditional FBA. Physiology-based pharmacokinetic (PBPK) models have been combined with FBA to study human liver metabolism, including hyperuricemia therapy, ammonia detoxification, and paracetamol-induced toxicity [110]. For ALA metabolism research, such integrated approaches could dynamically model the absorption, distribution, and metabolism of ALA and its metabolites throughout different tissues.
Table 2: Computational Approaches for Metabolic Flux Analysis
| Method | Key Features | Applications in ALA Research |
|---|---|---|
| Flux Balance Analysis (FBA) | Constraint-based, steady-state assumption, genome-scale | Predicting theoretical maximum yield of EPA/DHA from ALA |
| 13C-MFA | Uses isotopic labeling, more accurate than FBA | Measuring actual in vivo conversion rates in different tissues |
| Dynamic FBA | Incorporates time-varying constraints | Modeling postprandial ALA metabolism fluctuations |
| Machine Learning Integration | Pattern recognition in large datasets | Identifying biomarkers of ALA conversion efficiency |
| Kinetic Modeling | Enzyme-level parameters, dynamic | Predicting dose-response relationships for ALA supplementation |
Experimental Protocols for Flux Measurement
Stable Isotope Tracer Protocol for ALA Metabolism:
- Tracer Selection: Prepare 13C-uniformly labeled ALA or position-specific labeled tracers
- Administration: Introduce tracer to cell culture, tissue explants, or whole organisms via controlled delivery systems
- Sampling: Collect multiple time-point samples to capture metabolic dynamics
- Quenching: Rapidly quench metabolism using cold methanol or other cryogenic methods
- Metabolite Extraction: Implement dual-phase extraction to recover both polar and lipid metabolites
- Analysis: Utilize LC-MS/MS or GC-MS for isotopic labeling analysis with appropriate internal standards
- Data Processing: Correct for natural isotope abundance and calculate isotopomer distributions
- Flux Calculation: Apply computational models to estimate flux rates through metabolic networks
Integrated NMR-MS Protocol for Comprehensive Flux Analysis:
- Sample Preparation: Divide biological samples for parallel NMR and MS analysis
- NMR Analysis: Conduct 1H NMR with water suppression, and 13C NMR for positional enrichment
- MS Analysis: Perform targeted LC-MS/MS for specific pathway intermediates
- Data Integration: Combine NMR structural information with MS sensitivity data
- Pathway Reconciliation: Resolve discrepancies between platforms using statistical methods
- Flux Validation: Confirm computational predictions with experimental measurements
Biological and Technical Complexities in ALA Flux Measurement
Challenges Specific to ALA Metabolism
The measurement of metabolic fluxes in ALA metabolism presents unique challenges that extend beyond general technical limitations. Human studies indicate that the conversion efficiency of ALA to longer-chain omega-3 fatty acids is limited and highly variable between individuals [50] [107]. This conversion involves multiple enzymatic steps including Δ6-desaturase, elongation, and Δ5-desaturase reactions, with each step representing a potential bottleneck that complicates flux measurements.
Compartmentalization of metabolism between cellular organelles further complicates ALA flux analysis. The subcellular localization of desaturase and elongase enzymes in endoplasmic reticulum membranes creates challenges for measuring local substrate concentrations and reaction rates. Additionally, sex differences in ALA metabolism have been documented, with variations in conversion efficiency potentially related to hormonal influences on desaturase enzymes [50].
The competition for enzymatic machinery between fatty acid families represents another significant challenge. ALA competes with linoleic acid (LA) for the same desaturase and elongase enzymes, creating a metabolic network where fluxes are interdependent and influenced by the relative abundance of multiple substrates [50] [107]. This complexity necessitates sophisticated modeling approaches that can account for multi-substrate competition and allosteric regulation.
Tissue-Specific Flux Variations
ALA metabolism demonstrates significant tissue-specific variation in flux distribution, with the liver representing the primary site for conversion to EPA and DHA, while other tissues may preferentially oxidize ALA for energy production. This compartmentalization requires sophisticated modeling approaches that can account for inter-tissue metabolite exchange [50]. Recent research using integrated metabolic models of host and microbiome has revealed complex dependencies in metabolic pathways between different tissues and microbial communities [111].
Table 3: Technical Challenges in ALA Flux Measurement and Potential Solutions
| Challenge | Impact on Flux Measurements | Current Mitigation Strategies |
|---|---|---|
| Low Conversion Rates | Signal-to-noise issues in tracer studies | Use of highly enriched 13C tracers, sensitive detection methods |
| Multi-compartment Metabolism | Incomplete pathway mapping | Subcellular fractionation, computational modeling |
| Isotopic Dilution | Underestimation of flux rates | Compartment-specific metabolite measurements |
| Inter-individual Variation | Reduced statistical power | Larger cohort sizes, stratification by genetic factors |
| Dynamic Regulation | Steady-state assumptions violated | Multiple time-point sampling, dynamic modeling |
| Analytical Limitations | Incomplete metabolite coverage | Multi-platform approaches (NMR + MS) |
Future Perspectives and Emerging Solutions
Multi-Omics Integration and Advanced Modeling
The future of metabolic flux measurement lies in the integration of multiple omics technologies to create comprehensive models of metabolic regulation. Combining fluxomics with genomics, transcriptomics, proteomics, and metabolomics provides orthogonal validation and enhances the accuracy of flux predictions [106] [111]. For ALA metabolism research, this approach could identify genetic polymorphisms that influence enzyme activity and subsequently affect metabolic fluxes.
Machine learning integration with constraint-based models represents a promising frontier for flux analysis. ML algorithms can identify patterns in large datasets that may not be apparent through traditional biochemical approaches [110]. Recent studies have successfully applied principal component analysis, random forest algorithms, and artificial neural networks to analyze flux distributions and identify key regulatory nodes in metabolic networks [110].
Single-Cell Flux Analysis
Emerging technologies for single-cell metabolomics and flux analysis promise to overcome the limitations of population-averaged measurements. These approaches can reveal cell-to-cell heterogeneity in metabolic fluxes, which is particularly relevant for understanding tissue-specific ALA metabolism where different cell types may specialize in particular metabolic functions. While technical challenges remain in sensitivity and quantification, advances in microfluidics and mass spectrometry are rapidly progressing toward single-cell flux measurements.
The Scientist's Toolkit: Essential Research Reagents and Platforms
Table 4: Essential Research Tools for Metabolic Flux Studies
| Tool Category | Specific Examples | Application in Flux Studies |
|---|---|---|
| Isotopic Tracers | 13C-ALA, 2H-ALA, 15N-amino acids | Pathway tracing, flux quantification |
| Analytical Platforms | LC-MS/MS, GC-MS, NMR spectroscopy | Metabolite detection, quantification |
| Separation Techniques | HPLC, UPLC, GC columns | Metabolite separation before detection |
| Computational Tools | COBRA Toolbox, OptFlux, gapseq | Flux balance analysis, network modeling |
| Databases | MetaCyc, KEGG, Recon3D | Metabolic pathway references |
| Cell Culture Systems | Primary hepatocytes, engineered cell lines | Controlled environment for flux studies |
| Animal Models | Genetic knockouts, humanized models | In vivo flux analysis |
The measurement of metabolic fluxes in ALA metabolism confronts significant technical challenges spanning analytical limitations, computational complexities, and biological specificities. The integration of complementary analytical platforms like NMR and MS, combined with advanced computational modeling approaches including FBA and machine learning, provides a pathway toward more accurate flux quantification. As these technologies continue to evolve, particularly with advances in single-cell analysis and multi-omics integration, researchers will gain unprecedented insights into the metabolic fate of ALA and its health effects. Addressing these technical challenges is essential for resolving the ongoing controversies surrounding ALA metabolism and establishing evidence-based dietary recommendations for omega-3 fatty acid intake.
Therapeutic Efficacy and Comparative Analysis of ALA
Clinical Validation of ALA's Health Benefits
Alpha-linolenic acid (ALA) represents an essential omega-3 polyunsaturated fatty acid with demonstrated pleiotropic health benefits across numerous physiological domains. This comprehensive review synthesizes current clinical and preclinical evidence validating ALA's cardioprotective, neuroprotective, and metabolic effects. We examine the molecular mechanisms underpinning these benefits, including membrane integration, anti-inflammatory signaling, and metabolic modulation. Despite promising data, significant challenges remain in ALA bioavailability and conversion efficiency to longer-chain metabolites. This analysis provides researchers and drug development professionals with critical insights into ALA's validated clinical applications and future therapeutic potential, contextualized within the broader framework of fatty acid metabolism and health outcomes research.
Alpha-linolenic acid (ALA) is an 18-carbon, essential omega-3 polyunsaturated fatty acid classified as an essential fatty acid that must be obtained through dietary sources as humans cannot synthesize it endogenously [96] [47]. Chemically, ALA is characterized by three double bonds, with the first double bond located at the third carbon atom from the methyl end of the carbon chain [96]. This unique chemical structure confers distinct health-promoting properties, primarily through its role as a metabolic precursor to longer-chain omega-3 fatty acids, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [14] [96].
The metabolic conversion of ALA to its longer-chain derivatives occurs through a series of elongation and desaturation reactions, though this process exhibits significant limitations in humans. Crucially, sex differences in conversion to DHA have been identified, with generally limited capacity for this conversion in most individuals [14]. This constrained bioconversion has led to questions regarding whether ALA itself provides sufficient health benefits comparable to EPA and DHA, though emerging evidence confirms independent physiological roles for ALA beyond its function as a precursor [14] [112].
ALA serves as a critical structural component in cellular membranes, particularly in neurological tissues, where it contributes to neuronal structure and function, neurotransmitter synthesis, and overall cognitive health [96]. Beyond its structural roles, ALA exhibits potent anti-inflammatory properties through modulation of inflammatory mediator production and demonstrates significant effects on gene expression and metabolic processes [96] [112]. These multifaceted mechanisms underpin the diverse clinical benefits documented across experimental and clinical studies.
Mechanisms of Action: Molecular Pathways and Physiological Effects
Membrane Integration and Structural Modulation
ALA integrates into phospholipid bilayers of neural, cardiovascular, hepatic, and immune tissues, significantly enhancing membrane fluidity and signaling competency [112]. This integration facilitates optimal function of membrane-bound receptors and ion channels, creating a more responsive cellular environment. In neuronal tissues specifically, this membrane stabilization contributes to maintained cognitive function and neuroprotection against degenerative processes [96] [47].
Anti-inflammatory Signaling Pathways
ALA demonstrates potent anti-inflammatory effects primarily through nuclear factor kappa B (NF-κB) inhibition, resulting in subsequent reductions in cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) expression, and proinflammatory cytokines including tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) [112]. This NF-κB mediated mechanism represents a primary pathway through which ALA modulates inflammatory responses. Additionally, ALA contributes to rebalancing eicosanoid biosynthesis and promotes the production of specialized pro-resolving mediators (SPMs), actively facilitating inflammation resolution [112].
Antioxidant Defense Systems
Through its capacity to interrupt radical chain reactions and protect membrane lipids from oxidative damage, ALA provides significant antioxidant protection [112]. This activity complements its anti-inflammatory effects, creating a comprehensive cellular protection profile. The antioxidant mechanisms operate independently of classic antioxidant systems, providing complementary protection against oxidative stress.
Metabolic and Gene Expression Regulation
ALA influences metabolic processes through modulation of gene expression related to lipid metabolism, inflammatory pathways, and cellular signaling [96]. This regulatory capacity extends to metabolic processes in adipose tissue, where ALA has demonstrated effects on adipose inflammation and browning, contributing to improved metabolic parameters [112]. These metabolic effects position ALA as a significant modulator in conditions characterized by metabolic dysregulation.
Clinical Evidence: Quantitative Analysis of Health Outcomes
Cardiovascular Benefits
Clinical evidence supports ALA's cardioprotective effects through multiple mechanisms, including lipid modulation, anti-inflammatory activity, and anti-thrombotic effects. The data from prospective cohort studies and clinical interventions are summarized in Table 1.
Table 1: Clinical Evidence for ALA Cardiovascular Benefits
| Outcome Measure | Effect Size | Study Details | Reference |
|---|---|---|---|
| Stroke Risk | 37% reduction | Independent association with serum ALA levels in adult men | [47] |
| Carotid Atherosclerosis | Inverse association | Higher ALA intake associated with lower prevalence of carotid plaque | [47] |
| Anti-arrhythmic Effects | Significant reduction | Similar antiarrhythmic properties to EPA/DHA in animal models | [47] |
| Inflammatory Markers | Reduced proinflammatory cytokines | Relation to omega-6/omega-3 ratio reduction | [47] |
A large Dutch population-based cohort study involving over 20,000 adults revealed that ALA intake specifically lowered stroke risk, though no significant association with incident coronary heart disease was observed [47]. This suggests a particular cerebrovascular protective effect that merits targeted investigation.
Neurological and Cognitive Effects
ALA demonstrates significant neuroprotective properties in experimental models of neurological injury and degeneration. The pleiotropic mechanisms contributing to these benefits include enhancement of mature brain-derived neurotrophic factor (BDNF) expression, activation of neuronal background rectifying potassium channels leading to membrane hyperpolarization, and reduced glutamate excitotoxicity [47].
In Alzheimer's disease models, ALA has demonstrated specific anti-amyloidogenic effects through enhanced α-secretase cleavage of amyloid-β precursor protein (APP) by A Disintegrin and Metalloproteinase 10 (ADAM10) [113]. This mechanism represents a direct intervention in the amyloid pathway implicated in Alzheimer's pathology. Animal studies utilizing APP23/PS45 transgenic mouse models of Alzheimer's disease showed that ALA administration ameliorated amyloid plaque pathology and reduced learning and memory impairments [113]. The cognitive benefits were linked to BNIP3L-mediated mitophagy that facilitated ADAM10 maturation, promoting non-amyloidogenic processing of APP [113].
In stroke models, ALA administration has demonstrated protective effects against both focal and global ischemia, with mechanisms including modulation of NMDA receptor activation through membrane hyperpolarization [47]. This neuroprotective activity positions ALA as a promising candidate for both stroke prevention and recovery applications.
Metabolic and Inflammatory Modulation
Clinical studies have validated ALA's effects on metabolic parameters and inflammatory biomarkers, particularly in conditions characterized by metabolic dysregulation such as type 2 diabetes. The quantitative findings from meta-analyses of randomized controlled trials are summarized in Table 2.
Table 2: Meta-Analysis Findings on ALA Metabolic and Inflammatory Effects
| Parameter | Effect Size | Statistical Significance | Reference |
|---|---|---|---|
| Fasting Blood Glucose | WMD: -6.57 mg/dL | 95% CI: -11.91 to -1.23, P = 0.016 | [114] |
| HbA1c | WMD: -0.35% | 95% CI: -0.55 to -0.15, P = 0.004 | [114] |
| TNF-α | WMD: -1.57 pg/mL | 95% CI: -2.29 to -0.85, P < 0.05 | [114] |
| IL-6 | WMD: -1.15 pg/mL | 95% CI: -1.58 to -0.72, P < 0.001 | [114] |
| CRP | WMD: -0.31 mg/L | 95% CI: -0.47 to -0.16, P > 0.001 | [114] |
A systematic review and dose-response meta-analysis specifically examining oral ALA supplementation in type 2 diabetes patients found that each 500 mg/day increase in ALA supplementation significantly reduced HbA1c, body weight, C-reactive protein (CRP), fasting plasma glucose, and triglycerides [115]. The dose-response analysis indicated a linear decrement in body weight at ALA supplementation exceeding 600 mg/day, with a J-shaped effect observed for HbA1c reduction [115].
Experimental Models and Methodologies
In Vivo Animal Models
Transgenic Alzheimer's Mouse Model (APP23/PS45)
- Protocol: APP23/PS45 double transgenic mice with C57BL/6 background were administered ALA (5 mg/kg) or vehicle via intraperitoneal injection once daily for 4 months beginning at 2 months of age [113].
- Cognitive Assessment: Memory and learning function were evaluated using Morris water maze and contextual fear conditioning tests after the treatment period [113].
- Pathological Analysis: Brain tissues were analyzed for amyloid plaque burden, APP processing fragments, and ADAM10 expression levels using immunohistochemistry and Western blotting [113].
- Key Findings: ALA treatment significantly reduced amyloid plaque pathology and improved cognitive performance in transgenic mice while increasing ADAM10 expression and promoting non-amyloidogenic APP processing [113].
Ischemic Stroke Models
- Protocol: Rodent models of focal and global cerebral ischemia were administered ALA chronically prior to or following induced ischemic events [47].
- Outcome Measures: Infarct volume quantification, neurological deficit scoring, and assessment of neuroinflammatory markers were performed post-ischemia [47].
- Mechanistic Investigations: Electrophysiological studies examined ALA's effects on neuronal potassium channels and NMDA receptor activity [47].
- Key Findings: ALA pretreatment significantly reduced infarct volume and improved functional outcomes, with mechanisms involving membrane hyperpolarization and reduced glutamate excitotoxicity [47].
In Vitro Methodologies
APP Processing and Mitophagy Assays
- Cell Culture: 20E2 cell lines were treated with ALA concentration gradients to assess effects on APP proteolytic enzymes and metabolites [113].
- Inhibitor Studies: GI254023X (ADAM10 inhibitor) and CCCP (mitochondrial toxic drug) were employed to elucidate mechanisms of ALA action [113].
- Protein Analysis: Western blotting quantified expression levels of ADAM10, APP fragments, and mitophagy markers including BNIP3L [113].
- Key Findings: ALA increased ADAM10 expression and promoted non-amyloidogenic processing of APP to produce C83 fragments, effects dependent on BNIP3L-mediated mitophagy [113].
Research Reagent Solutions
Table 3: Essential Research Reagents for ALA Mechanistic Studies
| Reagent/Cell Line | Application | Function in ALA Research | |
|---|---|---|---|
| APP23/PS45 Transgenic Mice | In vivo Alzheimer's disease modeling | Express human APP751 with Swedish double mutation and PS1 with familial AD mutation; assess ALA effects on amyloid pathology and cognition | [113] |
| 20E2 Cell Line | In vitro APP processing studies | Evaluate ALA effects on APP metabolic pathway and secretase expression | [113] |
| GI254023X | ADAM10 inhibition | Specific inhibitor of ADAM10 α-secretase activity; mechanistic studies of APP processing | [113] |
| CCCP (Carbonyl Cyanide m-Chlorophenylhydrazone) | Mitochondrial function disruption | Mitochondrial toxic drug used to study mitophagy involvement in ALA mechanisms | [113] |
| Anti-ADAM10 Antibody | Protein detection and quantification | Western blot and immunohistochemical detection of ADAM10 expression changes | [113] |
| Anti-APP C-terminal Antibody (C20) | APP fragment analysis | Detection of APP and its C-terminal fragments (C83, C99) in processing studies | [113] |
| BNIP3L/NIX Antibodies | Mitophagy assessment | Detection and knockdown of BNIP3L to investigate mitophagy role in ADAM10 maturation | [113] |
Visualizing ALA Mechanisms and Experimental Approaches
ALA Metabolic Pathway and Physiological Mechanisms
Figure 1: ALA Metabolic Pathways and Physiological Mechanisms
Experimental Workflow for ALA Neuroprotection Studies
Figure 2: Experimental Workflow for ALA Neuroprotection Studies
Challenges and Future Research Directions
Bioavailability and Conversion Limitations
A significant challenge in ALA therapeutics is the limited conversion to EPA and DHA in humans. Research indicates that while stearidonic acid (SDA) is more readily converted to EPA than ALA, conversion of both ALA and SDA to DHA remains limited in most individuals [14]. This constrained metabolism potentially limits the efficacy of ALA supplementation for achieving clinical benefits associated with long-chain omega-3 fatty acids.
Strategies to enhance ALA bioavailability and conversion efficiency represent an important research direction. These may include co-administration with conversion-enhancing compounds, development of specialized delivery systems, or identification of genetic factors influencing conversion efficiency that could enable personalized supplementation approaches.
Clinical Translation Considerations
Recent analyses of neurodegenerative disease therapeutics highlight the critical importance of blood-brain barrier (BBB) penetration for central nervous system efficacy [116]. While ALA demonstrates favorable BBB penetration compared to many therapeutic compounds, optimizing brain bioavailability remains a research priority [113] [116]. The documented decline in endogenous ALA levels with aging further underscores the importance of supplementation strategies [113].
Future clinical trials would benefit from standardized dosing protocols, targeted patient populations based on metabolic characteristics, and combination approaches that address multiple pathological mechanisms simultaneously. The exploration of ALA as an adjunctive therapy alongside conventional treatments represents a promising direction for enhancing therapeutic outcomes across multiple disease states.
Comprehensive analysis of clinical and preclinical evidence confirms that alpha-linolenic acid delivers validated health benefits across neurological, cardiovascular, and metabolic domains. The mechanistic basis for these benefits involves membrane integration, anti-inflammatory signaling, NF-κB inhibition, and enhanced non-amyloidogenic APP processing through ADAM10 activation. Well-designed experimental models have provided robust methodological approaches for continued investigation of ALA's therapeutic potential.
Despite conversion limitations to long-chain metabolites, ALA demonstrates significant independent biological activity that supports its clinical application. Future research should address bioavailability optimization, precision medicine approaches based on individual metabolic variability, and exploration of synergistic combinations with complementary therapeutic agents. The accumulated evidence positions ALA as a viable nutraceutical intervention with particular promise in neurodegenerative and cerebrovascular conditions.
Omega-3 polyunsaturated fatty acids (PUFAs) are dietary nutrients essential for human health, playing critical roles in neurological function, inflammatory response, and cardiovascular homeostasis. The three primary omega-3 fatty acids include alpha-linolenic acid (ALA), found predominantly in plant sources, and eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are primarily derived from marine sources. While all are classified as omega-3s due to the location of their first double bond at the third carbon from the methyl end, their biochemical properties, metabolic fates, and physiological effects differ significantly. ALA (18:3n-3) is considered an essential fatty acid because humans cannot synthesize it endogenously and must obtain it from dietary sources. In contrast, EPA (20:5n-3) and DHA (22:6n-3) can be synthesized from ALA through a series of elongation and desaturation reactions, though this conversion is notoriously inefficient in humans. This whitepaper provides a comprehensive technical comparison between ALA and marine-derived EPA/DHA, examining their structural characteristics, metabolic pathways, bioavailability, and relative efficacy in addressing various health outcomes, with particular emphasis on the implications for research and therapeutic development.
Biochemical Properties and Metabolic Pathways
The fundamental differences between ALA and marine-derived omega-3s begin with their chemical structures and subsequent metabolic handling within the human body. Understanding these distinctions is crucial for interpreting their differential biological activities.
ALA is a short-chain omega-3 fatty acid containing 18 carbon atoms with three double bonds. It is predominantly found in plant-based sources including flaxseed, chia seeds, walnuts, and canola oil. Specifically, flaxseed contains approximately 2.4 grams per tablespoon, chia seeds provide 5 grams per ounce, and walnuts offer 2.6 grams per ounce [117]. Marine-derived EPA and DHA are long-chain polyunsaturated fatty acids. EPA contains 20 carbon atoms and five double bonds, while DHA is a 22-carbon compound with six double bonds. These are obtained directly from marine sources including fatty fish (salmon, mackerel, sardines), fish oils, krill oil, and algal oils. A 3-ounce serving of farmed Atlantic salmon provides approximately 733 mg of EPA and 935 mg of DHA, alongside only 126 mg of ALA [117]. Plant sources are virtually devoid of pre-formed EPA and DHA, while marine sources typically contain only trace amounts of ALA, establishing a clear dichotomy in the dietary provision of these fatty acid classes [118].
Metabolic Conversion of ALA to EPA and DHA
The metabolic pathway for converting ALA to the long-chain EPA and DHA involves a series of elongation and desaturation steps, primarily occurring in the liver. This process is mediated by the enzymes delta-6-desaturase (D6D), elongase-5, and delta-5-desaturase (D5D). ALA is first converted to stearidonic acid (SDA, 18:4n-3) by D6D, then elongated to eicosatetraenoic acid (20:4n-3), and subsequently desaturated by D5D to form EPA (20:5n-3). Further elongation and desaturation, followed by peroxisomal beta-oxidation, are required to produce DHA (22:6n-3) [118].
However, this conversion pathway is remarkably inefficient in humans. Comprehensive studies using deuterated ALA and gas chromatography-mass spectrometry analysis have demonstrated that conversion efficiencies range from approximately 5% to 15% for ALA to EPA combined, but only 0% to 8% on average for ALA to DHA conversion [118]. Significant demographic variations exist, with premenopausal women converting ALA to EPA at approximately 21% and to DHA at 9%, while men exhibit significantly lower conversion rates of approximately 8% for EPA and 0-4% for DHA [117]. This inefficiency stems from several factors: the competitive inhibition by high dietary omega-6 fatty acids (particularly linoleic acid) for the same desaturase and elongase enzymes, the preferential use of ALA for beta-oxidation and energy production rather than elongation, and genetic polymorphisms in the desaturase enzymes [118] [117]. Consequently, dietary ALA is a substantially ineffective substrate for increasing tissue levels of DHA, the physiologically essential omega-3 for neurological function [118].
Diagram 1: ALA metabolic pathway with inhibition points. The conversion of ALA to EPA and DHA is limited by multiple enzymatic steps and competitively inhibited by high omega-6 fatty acid intake.
Bioavailability and Tissue Distribution
The bioavailability of omega-3 fatty acids varies considerably between ALA and marine-derived EPA/DHA, influenced by their chemical forms, delivery systems, and metabolic handling.
Absorption Efficiency and Chemical Forms
Marine-derived omega-3s demonstrate variable bioavailability depending on their chemical forms. Acute and chronic human studies have established that bioavailability follows a consistent hierarchy: non-esterified fatty acids (NEFA) > phospholipids (PL) > re-esterified triacylglycerols (rTAG) > unmodified triacylglycerols (TAG) > ethyl esters (EE) [119]. Krill oil, which contains omega-3s primarily in phospholipid form, and algal oils demonstrate differing absorption kinetics compared to conventional fish oils. However, it is noteworthy that significant differences observed in acute bioavailability studies (e.g., faster absorption rates) often do not translate into substantial long-term impacts in chronic supplementation studies, raising questions about the clinical relevance of acute pharmacokinetic differences [119].
ALA absorption occurs efficiently from dietary sources, but its subsequent metabolic fate differs fundamentally from pre-formed EPA and DHA. Unlike marine omega-3s, which are directly incorporated into tissue membranes, ALA is predominantly oxidized for energy or undergoes inefficient conversion to longer-chain metabolites. When ALA is consumed, even in gram quantities, it fails to significantly increase DHA levels in critical tissues such as brain tissue or human breast milk, underscoring the profound limitations of ALA as a precursor for DHA accretion [118].
Tissue Distribution and Physiological Roles
The tissue distribution of omega-3 fatty acids reveals their distinct physiological roles. DHA is the predominant omega-3 fatty acid in the brain, constituting over 40% of the polyunsaturated fatty acids in gray matter and is particularly concentrated in neuronal membranes and synaptic junctions. Similarly, the retina contains very high levels of DHA, which is essential for visual acuity. In these tissues, the omega-3 fatty acid profile consists almost exclusively of DHA, with ALA found only in trace amounts regardless of dietary ALA intake [118] [117]. This selective incorporation highlights the physiological essentiality of pre-formed DHA for neurological and visual function.
EPA demonstrates different distribution patterns, with higher concentrations in immune cells, platelets, and the vascular endothelium, correlating with its roles in inflammatory mediation and vascular function. While ALA is widely distributed throughout body tissues, it does not preferentially accumulate in neural tissues and is primarily utilized as a metabolic fuel rather than a structural component [118].
Table 1: Bioavailability and Tissue Distribution Characteristics
| Parameter | ALA (Plant Source) | EPA/DHA (Marine Sources) |
|---|---|---|
| Primary Chemical Forms in Diet | Triacylglycerols in plants | TAG, PL, EE in supplements; TAG/PL in fish |
| Absorption Efficiency | Efficient absorption but poor conversion to long-chain metabolites | Variable by form: NEFA > PL > rTAG > TAG > EE |
| Conversion Rate to EPA | 8% in men, 21% in women | Pre-formed, no conversion required |
| Conversion Rate to DHA | 0-4% in men, 9% in women | Pre-formed, no conversion required |
| Brain Concentration | Trace amounts only | High (DHA constitutes >40% of gray matter PUFAs) |
| Primary Metabolic Fate | β-oxidation for energy; limited elongation | Membrane incorporation; eicosanoid synthesis |
Comparative Health Outcomes Efficacy
The structural and metabolic differences between ALA and marine-derived omega-3s translate into significant variations in their efficacy across multiple health domains.
Neurological and Cognitive Health
DHA is unequivocally recognized as the physiologically essential omega-3 fatty acid for neurological development and function. It is integral to the structure and fluidity of neuronal cell membranes, synaptic transmission, and neuroprotection. Clinical studies demonstrate that DHA supplementation, not ALA, improves mental development indices in infants and supports cognitive performance across the lifespan [118]. While ALA consumption may offer indirect neurological benefits through its minimal conversion to DHA, the effect is substantially inferior to direct EPA/DHA consumption. The limited ability of ALA to cross the blood-brain barrier and its negligible presence in brain tissue further underscore its limitations for neurological applications [118] [117].
Research on cognitive decline and neurodegenerative diseases suggests that dietary and supplemental marine omega-3s may have a protective effect against cognitive decline in healthy individuals, though evidence supporting benefits for those already diagnosed with Alzheimer's disease remains limited [120]. The presence of pre-formed DHA, rather than ALA, in neuronal membranes is critical for maintaining membrane integrity and facilitating neuronal signaling throughout the lifespan.
Cardiovascular Health Outcomes
Both ALA and marine-derived omega-3s demonstrate cardioprotective effects, though through partially distinct mechanisms and with differing magnitudes of benefit.
Table 2: Cardiovascular Health Effects Comparison
| Cardiovascular Parameter | ALA Impact | EPA/DHA Impact |
|---|---|---|
| Triglyceride Reduction | Modest effects | ~15% reduction [120] |
| Sudden Cardiac Death Risk | Up to 40% reduction in women when marine intake low [118] | 45% reduction [117] |
| Fatal Myocardial Infarction | ~20% risk reduction with 1.2g/day additional ALA [118] | Significant risk reduction |
| Overall Mortality | Moderate reduction | 20% reduction [117] |
| Blood Pressure | Limited evidence | Modest reduction at doses >800mg/day [121] |
| Primary Mechanism | Possible conversion to EPA; alternative pathways | Anti-arrhythmic; triglyceride lowering; anti-inflammatory |
Epidemiological evidence suggests that ALA consumption is associated with an approximate 20% lower relative risk of fatal heart disease for high versus low intakes [118]. Intervention studies indicate that increasing ALA intake by 1.2 g/day may decrease the risk of fatal coronary heart disease by approximately 20% [118]. However, the protective effects of ALA appear most pronounced when background consumption of marine omega-3s is low (below 100 mg/day of combined EPA/DHA) [118]. This suggests that ALA may serve as a partial substitute when marine sources are inadequate, but cannot fully replicate the benefits of pre-formed EPA and DHA.
Marine-derived EPA and DHA demonstrate more robust and multifaceted cardioprotective effects. The GISSI-Prevenzione study demonstrated that supplementation with approximately 900 mg/day of combined EPA/DHA in post-myocardial infarction patients significantly reduced sudden cardiac deaths compared to placebo-controlled groups [118]. Multiple meta-analyses confirm that marine omega-3 supplementation reduces the risk of coronary events and coronary death, with benefits particularly evident at higher intake levels (1,000 mg/day or more) [121] [120]. These findings have led the American Heart Association to recommend at least two servings of fatty fish per week for cardiovascular risk reduction, and approximately 900 mg/day of combined EPA/DHA for those with established coronary disease [118].
Anti-inflammatory and Other Health Effects
The anti-inflammatory properties of marine-derived omega-3s substantially exceed those of ALA. EPA and DHA serve as substrates for the production of specialized pro-resolving mediators (SPMs) including resolvins, protectins, and maresins, which actively resolve inflammatory processes. Clinical evidence demonstrates that fish oil supplementation (3-4 g/day of EPA/DHA) significantly reduces inflammatory markers including TNF (decreased by 24.9%), RANTES (reduced by 12.1%), and MIP-1β (lowered by 12.5%) in adults with abdominal obesity [117]. In rheumatoid arthritis, marine omega-3s significantly reduce disease activity and inflammation, whereas ALA provides only about one-eighth of the anti-inflammatory potency of EPA and DHA [117].
Other health domains also demonstrate the superiority of marine sources. For eye health, DHA is concentrated in retinal photoreceptors and essential for visual acuity, benefits not achievable through ALA consumption alone [118]. Regarding cancer risk, some evidence suggests a potential association between high ALA intake and increased risk of advanced prostate cancer, whereas marine omega-3s may reduce this risk [118]. However, the authors note that factors other than ALA in food sources may contribute to these epidemiological observations.
Research Methodologies and Experimental Protocols
Robust experimental methodologies are essential for accurately assessing omega-3 status, metabolism, and biological effects in clinical and preclinical research.
Assessment of Omega-3 Status and Bioavailability
Stable Isotope Tracer Methodology: The gold standard for evaluating ALA conversion efficiency involves administration of deuterated or carbon-13 labeled ALA followed by serial blood sampling and gas chromatography-mass spectrometry (GC-MS) analysis. This methodology enables precise quantification of newly synthesized EPA and DHA, avoiding contamination from pre-existing pools. The standard protocol involves oral administration of 50 mg of deuterated ALA per kg body weight, with blood samples collected at baseline, 2, 4, 6, 8, 12, 24, 48, 72, and 168 hours post-administration. Plasma lipids are extracted, fractionated by thin-layer chromatography, transesterified to fatty acid methyl esters, and analyzed by GC-MS with selective ion monitoring for the deuterated species [118].
Omega-3 Index Determination: The Omega-3 Index, defined as the percentage of EPA + DHA in red blood cell membranes, provides a validated biomarker of long-term omega-3 status and cardiovascular risk. The standardized protocol involves collection of whole blood in EDTA tubes, isolation of red blood cells by centrifugation, direct transesterification with sulfuric acid in methanol, and GC analysis of fatty acid methyl esters using a highly polar capillary column (e.g., CP-Sil 88) with flame ionization detection. Results are expressed as the percentage of total identified fatty acids after response factor correction [121].
Acute Bioavailability Studies: Single-dose pharmacokinetic studies typically administer different chemical forms of omega-3s (EE, TAG, rTAG, PL) to fasting participants, with serial blood sampling over 24-72 hours. Plasma concentrations of EPA and DHA are quantified, and pharmacokinetic parameters (Cmax, Tmax, AUC) are calculated. Chronic supplementation studies extend over 8-24 weeks, measuring changes in erythrocyte membrane composition or the Omega-3 Index as primary outcomes [119].
Experimental Models for Efficacy Assessment
Inflammatory Marker Analysis: Clinical trials evaluating anti-inflammatory effects typically employ randomized, placebo-controlled designs with 8-24 week intervention periods. Key inflammatory biomarkers include high-sensitivity C-reactive protein (hs-CRP), tumor necrosis factor-alpha (TNF-α), interleukins (IL-6, IL-1β), and specialized pro-resolving mediators. Sample processing involves collection of serum or plasma under standardized conditions, immediate freezing at -80°C, and analysis using high-sensitivity ELISA or multiplex immunoassay platforms [117].
Cardiovascular Function Assessment: Comprehensive cardiovascular assessment includes endothelial function measurement via flow-mediated dilation (FMD), arterial stiffness via pulse wave velocity, cardiac rhythm analysis via 24-hour Holter monitoring, and lipid profiling through standardized clinical chemistry methods. These methodologies require strict standardization of participant conditions (fasting, caffeine abstinence, time of day) and environmental controls to minimize variability [118] [120].
Neurological Function Evaluation: Cognitive assessment batteries for omega-3 research typically include tests of memory (e.g., Rey Auditory Verbal Learning Test), executive function (e.g., Trail Making Test), processing speed, and overall cognitive screening (e.g., MMSE, MoCA). For neuroimaging studies, magnetic resonance spectroscopy can quantify DHA content in brain tissues, while functional MRI assesses neuronal activation patterns in response to omega-3 supplementation [120].
Diagram 2: Experimental workflow for omega-3 research. Comprehensive assessment requires standardized protocols from study design through laboratory analysis to clinical endpoint evaluation.
The Scientist's Toolkit: Essential Research Reagents and Methodologies
Table 3: Essential Research Reagents and Analytical Tools
| Reagent/Instrument | Application | Technical Specifications | Research Utility |
|---|---|---|---|
| Deuterated ALA (d5-ALA) | Metabolic tracer studies | 98% isotopic purity; 50 mg/kg body weight dosage | Quantifies ALA conversion efficiency to EPA/DHA in humans |
| GC-MS with Polar Column | Fatty acid analysis | CP-Sil 88 or equivalent column; electron impact ionization | Precise quantification of fatty acid profiles and tracer incorporation |
| Omega-3 Index Methodology | Cardiovascular risk assessment | RBC collection; direct transesterification; GC-FID analysis | Standardized biomarker for long-term omega-3 status |
| Specialized Pro-Resolving Mediator Standards | Inflammation resolution studies | LC-MS/MS detection; authentic SPM standards (RvD1, RvE1, etc.) | Measures bioactive metabolites of EPA/DHA in inflammatory processes |
| Fatty Acid Standard Mixtures | Analytical calibration | GLC-463 to GLC-744 (Nu-Chek Prep) with certified concentrations | Essential for accurate quantification and method validation |
| Phospholipase A2 Assay | Inflammatory biomarker | ELISA-based detection of Lp-PLA2 mass and activity | Assesses vascular inflammation and response to omega-3 interventions |
| Endothelial Function Imaging | Vascular health assessment | High-resolution ultrasound with FMD software | Non-invasive assessment of vascular endothelial function |
The comparative analysis of ALA versus marine-derived omega-3s reveals fundamental differences in their biochemical properties, metabolic handling, and clinical efficacy. While ALA serves as an essential dietary fatty acid, its inefficient conversion to physiologically active EPA and particularly DHA substantially limits its capacity to replicate the benefits of pre-formed marine omega-3s. The evidence demonstrates that marine-derived EPA and DHA provide superior efficacy for neurological development and function, cardiovascular risk reduction, and inflammation resolution. ALA may offer partial cardioprotective benefits, particularly when marine omega-3 intake is inadequate, but cannot substitute for direct EPA/DHA consumption, especially for neurological health. Future research should prioritize the development of more sensitive biomarkers of omega-3 status and metabolism, standardized protocols for bioavailability assessment, and precision nutrition approaches to identify individual factors influencing ALA conversion efficiency. These advancements will enable more targeted dietary recommendations and therapeutic applications of omega-3 fatty acids based on their distinct metabolic and functional characteristics.
Biomarker Validation for ALA Status and Metabolism
The validation of biomarkers for alpha-linolenic acid (ALA) status and metabolism represents a critical component of nutritional science and clinical research, enabling the precise assessment of ALA intake, bioavailability, metabolic conversion, and relationship to health outcomes. ALA, an essential omega-3 polyunsaturated fatty acid derived primarily from plant sources, serves as a metabolic precursor to longer-chain fatty acids like eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) through a series of elongation and desaturation reactions [31]. The accurate measurement of ALA status through validated biomarkers provides invaluable insights into its role in reducing risk of chronic diseases, including cardiovascular disease and certain cancers [43] [122]. This technical guide examines the core principles, methodologies, and applications of biomarker validation for ALA status, providing researchers and drug development professionals with a comprehensive framework for conducting rigorous ALA-focused research within the context of a broader investigation into ALA metabolism and health benefits.
Biomarker Types for ALA Status Assessment
Research utilizes distinct but complementary biomarker types to assess ALA status, each with specific applications, advantages, and limitations in the context of validation studies.
Table 1: Biomarker Types for Assessing ALA Status and Metabolism
| Biomarker Category | Specific Biomarkers | Research Applications | Key Advantages | Validation Considerations |
|---|---|---|---|---|
| Dietary Intake Biomarkers | Food Frequency Questionnaires (FFQs), Dietary Records | • Population-level intake assessment• Association studies with health outcomes | • Captures habitual intake• Practical for large cohorts | • Subject to recall bias• Database limitations for ALA content |
| Tissue Concentration Biomarkers | Plasma/Serum phospholipids, Erythrocyte membranes, Adipose tissue | • Objective status assessment• Metabolic conversion studies• Dose-response relationships | • Free from recall bias• Reflects internal dose• Represents longer-term status | • Pre-analytical handling critical• Requires specialized analytical methods |
| Metabolic Conversion Biomarkers | EPA:DHA ratios, LA:ALA ratios, Desaturation indices | • Elongation/desaturation efficiency• Genetic and dietary influences on metabolism | • Reveals functional metabolic capacity• Identifies metabolic phenotypes | • Affected by multiple factors• Requires precise analytical methods |
The selection of appropriate biomarkers follows a validation continuum that progresses from discovery through validation to implementation phases [123]. In the discovery phase, researchers analyze smaller sample sets to identify potential biomarker candidates, such as specific lipid fractions or ratios that correlate with ALA intake or metabolic status. The pre-validation phase employs cross-validation methods, such as the holdout method, to eliminate spurious positive biomarkers before proceeding to large-scale validation [123]. The final validation phase involves large-scale corroboratory studies using independent sample sets from target populations, typically including healthy controls, disease groups, and groups with comorbid conditions to determine the biomarker's capability across diverse physiological states [123].
Analytical Methodologies for ALA Biomarker Quantification
Chromatographic Techniques for Fatty Acid Analysis
Gas chromatography (GC) coupled with flame ionization detection (FID) or mass spectrometry (MS) represents the gold standard for precise quantification of ALA and its metabolites in biological samples. The methodology involves lipid extraction from samples (plasma, erythrocytes, or adipose tissue) using organic solvents such as chloroform:methanol (2:1 v/v) via a modified Folch extraction [31], followed by transesterification to generate fatty acid methyl esters (FAMEs) for analysis. The critical validation parameters for chromatographic methods include linearity (typically demonstrating R² > 0.99 across expected concentration ranges), precision (intra- and inter-assay CV < 10%), accuracy (85-115% recovery of spiked standards), and limit of quantification (sufficient to detect physiological ALA levels, generally <0.05% of total fatty acids) [43] [122] [31].
Stable Isotope Tracer Methods
The use of [13C]-labeled ALA enables precise quantification of ALA conversion kinetics to EPA and DHA in vivo. In this approach, [13C]ALA is administered to study participants, and temporal changes in [13C] enrichment in downstream metabolites are quantified using gas chromatography-combustion-isotope ratio mass spectrometry (GC-C-IRMS) [31]. This method provides direct measurement of ALA metabolic flux through the elongation and desaturation pathway, offering insights into factors that influence conversion efficiency, such as the ratio of dietary linoleic acid (LA) to ALA [31]. Experimental evidence indicates that the LA:ALA ratio significantly impacts conversion rates, with maximum EPA and DHA production observed at a 1:1 ratio in hepatocyte models [31].
Enzyme-Linked Immunosorbent Assay (ELISA) for Protein Biomarkers
While not directly measuring ALA, ELISA methods can quantify protein biomarkers associated with ALA status and metabolism, such as peroxisome proliferator-activated receptors (PPARs), sterol regulatory element binding proteins (SREBPs), and desaturase enzymes [124] [125]. The sandwich ELISA format, which provides high sensitivity and specificity, is particularly valuable for detecting low-abundance regulatory proteins involved in ALA metabolism [125]. The assay employs a capture antibody specific to the target protein immobilized on a microplate surface, followed by antigen binding and detection with an enzyme-linked antibody that generates a measurable signal upon substrate addition [124] [125]. Critical validation parameters for ALA-related protein biomarker ELISAs include standard curve performance, dilutional linearity, spike recovery, and cross-reactivity assessment to ensure specific detection of target proteins amidst complex biological matrices [125].
Biomarker Validation Framework and Metrics
The validation of ALA biomarkers follows a structured framework assessing multiple performance characteristics essential for research and clinical application.
Table 2: Core Validation Metrics for ALA Status Biomarkers
| Validation Parameter | Assessment Method | Acceptance Criteria | Application to ALA Biomarkers |
|---|---|---|---|
| Analytical Sensitivity | Limit of Detection (LOD), Limit of Quantification (LOQ) | LOD: Signal-to-noise > 3:1LOQ: CV < 20% at lower limit | Critical for detecting low-abundance ALA metabolites in tissue samples |
| Analytical Specificity | Cross-reactivity with similar fatty acids, Matrix interference | < 5% cross-reactivity with analogous fatty acids (e.g., γ-linolenic acid) | Essential to distinguish ALA from other C18 fatty acids in chromatographic methods |
| Precision | Intra-assay CV%, Inter-assay CV% | CV < 10% for within-run, < 15% for between-run | Evaluates consistency of ALA quantification across multiple measurements |
| Accuracy | Recovery of spiked standards, Comparison with reference methods | 85-115% recovery of known ALA concentrations | Validates ALA measurement against certified reference materials |
| Linearity | Serial dilution of samples across expected range | R² > 0.98 across measuring range | Ensures proportional ALA detection across physiological concentrations |
| Stability | Freeze-thaw cycles, Short-term and long-term storage | < 15% change after 3 freeze-thaw cycles | Determines appropriate handling conditions for ALA-containing samples |
The validation process must also address practical challenges specific to ALA biomarker research. Pre-analytical variables including sample collection methods, anticoagulant choice, processing time, and storage conditions significantly impact ALA stability in biological samples [123] [43]. Biological variability introduces additional complexity, as ALA levels fluctuate in response to recent intake, circadian rhythms, and individual metabolic differences [43] [122]. Incorporating multiple sample collections and standardizing sampling conditions relative to meals helps account for this variability. The validation framework should also consider population-specific factors such as age, sex, genetic polymorphisms in desaturase enzymes (FADS1/2), and comorbid conditions that influence ALA metabolism and biomarker performance [122] [31].
Experimental Models for ALA Biomarker Validation
In Vitro Models
HepG2 human hepatoma cells serve as a validated in vitro system for investigating ALA metabolism and validating metabolic biomarkers [31]. The experimental protocol involves culturing HepG2 cells in standardized medium, followed by incubation with [13C]ALA at varying LA:ALA ratios (e.g., 9:1, 4:1, 1:1, 1:0, and 0:1) to model different dietary patterns [31]. After incubation (typically 24 hours), cells are harvested, lipids extracted, and ALA conversion to EPA and DHA quantified using GC-MS. This model enables precise control over substrate concentrations and environmental factors, allowing researchers to investigate specific aspects of ALA metabolism, including the competitive inhibition between n-6 and n-3 fatty acids for desaturase enzymes [31]. Transcript levels of genes encoding delta-5 desaturase (D5D), delta-6 desaturase (D6D), peroxisome proliferator-activated receptor alpha (PPARα), and sterol regulatory element binding protein 1c (SREBP-1c) provide complementary data on regulatory mechanisms [31].
Clinical Trial Designs for Biomarker Validation
Prospective cohort studies represent the primary clinical design for validating ALA biomarkers against hard health endpoints [43] [122]. The systematic review and meta-analysis by (2021) exemplifies this approach, incorporating 41 prospective studies totaling 1,197,564 participants with follow-up periods ranging from 2 to 32 years [43]. This study demonstrated that higher ALA intake was significantly associated with lower risk of death from all causes (pooled RR 0.90), cardiovascular disease (pooled RR 0.92), and coronary heart disease (pooled RR 0.89), supporting the validity of dietary ALA biomarkers for predicting cardiovascular outcomes [43]. For tissue biomarkers, nested case-control studies within larger cohorts provide an efficient design for validating ALA status biomarkers, with cases (e.g., colorectal cancer) matched to controls for analysis of pre-diagnostic ALA levels in blood or adipose tissue [122].
Research Reagent Solutions for ALA Biomarker Studies
Table 3: Essential Research Reagents for ALA Biomarker Validation
| Reagent Category | Specific Examples | Research Application | Technical Considerations |
|---|---|---|---|
| Chromatography Standards | [13C]ALA methyl esters, Unlabeled ALA standards, Internal standards (e.g., heptadecanoic acid) | GC-MS/FID quantification, Stable isotope tracer studies | Isotopic purity > 99%, Chemical stability, Concentration verification |
| Antibodies for Metabolic Enzymes | Anti-D5D, Anti-D6D, Anti-PPARα, Anti-SREBP-1c | ELISA, Western blot, Immunohistochemistry | Species specificity, Epitope characterization, Cross-reactivity validation |
| Cell Culture Reagents | HepG2 cell line, DMEM medium, Fetal calf serum, Antibiotic solutions | In vitro metabolism studies, Pathway analysis | Serum fatty acid composition, Passage number effects, Mycoplasma testing |
| ELISA Components | Coating antibodies, Detection antibodies, Enzyme conjugates (HRP, AP), Substrates (TMB, ABTS) | Protein biomarker quantification, Receptor expression | Matched antibody pairs, Signal optimization, Interference minimization |
| Sample Preparation Kits | Lipid extraction kits, Methylation reagents, Solid-phase extraction columns | Sample processing, Fatty acid methylation | Recovery efficiency, Contamination prevention, Reaction completeness |
Signaling Pathways in ALA Metabolism
The following diagram illustrates key signaling pathways and regulatory mechanisms involved in ALA metabolism and biomarker expression:
Figure 1: ALA Metabolic Pathways and Regulation
Biomarker Validation Workflow
The following diagram outlines the comprehensive workflow for validating ALA status biomarkers from discovery through clinical implementation:
Figure 2: ALA Biomarker Validation Workflow
Applications and Interpretation of Validated ALA Biomarkers
Validated ALA biomarkers find application across multiple research domains, each requiring specific interpretive frameworks. In nutritional epidemiology, ALA biomarkers help resolve inconsistencies between dietary intake data and health outcomes, as demonstrated in a 2022 meta-analysis of prospective cohort studies that found blood levels of ALA were inversely associated with colorectal cancer risk (summary RR 0.83) while dietary ALA showed no significant association [122]. This discrepancy highlights the superior accuracy of tissue biomarkers over dietary recall methods and their value in elucidating true diet-disease relationships. In clinical intervention studies, validated ALA biomarkers enable precise quantification of dose-response relationships and metabolic conversion efficiency, informing dietary recommendations and therapeutic applications. For drug development, ALA biomarkers serve as pharmacodynamic endpoints for lipid-modifying therapies and anti-inflammatory agents, providing mechanistic insights into compound effects on fatty acid metabolism and related signaling pathways [31].
The interpretation of ALA biomarker data must account for several contextual factors. The n6/n3 fatty acid ratio significantly influences ALA metabolism, with in vitro evidence indicating that a LA:ALA ratio of 1:1 maximizes EPA and DHA production compared to higher ratios more typical of Western diets [31]. Genetic polymorphisms, particularly in the FADS gene cluster encoding desaturase enzymes, create distinct metabolic phenotypes that modulate ALA conversion efficiency and biomarker responses [122]. Compartmental differences in ALA distribution and metabolism mean that biomarkers from different tissues (plasma, erythrocytes, adipose) reflect varying exposure timeframes and metabolic pools, requiring careful selection based on study objectives [43] [122]. Finally, health status and comorbidities influence ALA biomarker levels and interpretation, as conditions like metabolic syndrome, inflammatory disorders, and liver dysfunction alter fatty acid metabolism and clearance rates [43].
The validation of biomarkers for ALA status and metabolism requires a systematic, multi-phase approach that addresses analytical performance, biological variability, and clinical relevance. Through the application of robust chromatographic methods, stable isotope tracers, and molecular techniques, researchers can develop validated biomarkers that accurately reflect ALA intake, metabolic status, and conversion efficiency. These biomarkers provide critical tools for advancing our understanding of ALA's health effects, elucidating metabolic pathways, and developing targeted nutritional and therapeutic interventions. As research progresses, the integration of novel analytical platforms with established validation frameworks will further enhance our capacity to precisely quantify ALA status and its relationship to human health.
In cancer research, the acronym "ALA" refers to two distinct bioactive molecules with unique properties and mechanisms of action: alpha-linolenic acid (an omega-3 polyunsaturated fatty acid) and alpha-lipoic acid (a potent antioxidant and mitochondrial cofactor). This semantic distinction is crucial for accurate scientific discourse, as these compounds exhibit fundamentally different biological activities, metabolic pathways, and therapeutic implications in oncological contexts.
Alpha-linolenic acid (α-linolenic acid, ALA) is an essential n-3 polyunsaturated fatty acid (n-3 PUFA) that serves as a metabolic precursor to longer-chain n-3 PUFAs such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), though this conversion is inefficient in humans [126]. It is primarily obtained through dietary sources such as flaxseed, perilla seed, chia seed, and rapeseed [126]. In contrast, alpha-lipoic acid (α-lipoic acid, ALA; also known as thioctic acid) is a sulfur-containing compound synthesized in mitochondria that functions as an essential cofactor for several mitochondrial enzyme complexes, including pyruvate dehydrogenase and α-ketoglutarate dehydrogenase [77] [127]. While both compounds have demonstrated anticancer properties in experimental models, their mechanisms of action, molecular targets, and research applications differ significantly, necessitating careful differentiation in scientific communication.
This review systematically examines the disease-specific evidence for both ALA compounds across various cancer types, highlighting molecular mechanisms, experimental findings, and potential therapeutic applications for researchers and drug development professionals.
Alpha-Linolenic Acid (ALA): Anticancer Evidence and Mechanisms
Epidemiological and Clinical Evidence
The relationship between alpha-linolenic acid intake and cancer risk has been investigated in numerous prospective cohort studies, with particular focus on colorectal cancer (CRC). A comprehensive dose-response meta-analysis of prospective cohort studies published in 2022 provided compelling evidence regarding the association between ALA biomarkers and CRC risk [122].
Table 1: Summary of Meta-Analysis Findings on ALA and Colorectal Cancer Risk
| Exposure Assessment Method | Number of Studies | Summary Risk Ratio (RR) | 95% Confidence Interval | Dose-Response Relationship |
|---|---|---|---|---|
| Dietary intake questionnaires | 11 | 1.03 | 0.97–1.10 | Non-significant (p = 0.24) |
| Blood biomarkers | 4 | 0.83 | 0.69–0.99 | Significant (10% risk reduction per 0.1% increase) |
| Adipose tissue biomarkers | 1 | 0.83 | 0.69–0.99 | Not assessed |
This meta-analysis, which included 12,239 CRC cases among 861,725 participants with a median follow-up of 9.3 years, revealed a critical methodological insight: while dietary recall assessments showed no significant association, objective biomarker measurements demonstrated a consistent protective effect [122]. Each 0.1% increase in blood ALA levels was associated with a 10% reduction in CRC risk (RR: 0.90, 95% CI: 0.80–0.99), suggesting that circulating ALA levels may provide a more accurate reflection of biological activity than dietary recall alone [122].
Molecular Mechanisms of Action
Alpha-linolenic acid exerts antitumor effects through multiple interconnected molecular pathways, as demonstrated in various cancer models:
Inhibition of Proliferation: In esophageal cancer cell lines (OE19 and OE33), ALA alone or combined with oleic acid inhibited proliferation by regulating the AMPK/S6 axis, activating tumor suppressor genes p53, p21, and p27 [126]. In renal cell carcinoma, ALA dose-dependently inhibited proliferation by significantly increasing PPAR-γ activity and gene expression while suppressing COX-2 [126].
Induction of Apoptosis: ALA promotes apoptosis in cancer cells by modulating the expression of apoptotic regulators. In colorectal cancer models, ALA has been shown to activate caspase-3, a key executioner protease in the apoptotic cascade [126].
Anti-inflammatory Effects: ALA exerts potent anti-inflammatory activity by suppressing pro-inflammatory mediators, including prostaglandins, leukotrienes, COX-2, PGE2, IL-1β, and IL-6, across multiple cancer types including prostate, breast, and pancreatic cancers [126].
Inhibition of Metastasis and Angiogenesis: In breast cancer models, ALA suppressed metastasis through downregulation of Twist1, a key transcription factor in epithelial-mesenchymal transition [126].
Cancer-Type Specific Effects
Table 2: Anticancer Effects of Alpha-Linolenic Acid Across Cancer Types
| Cancer Type | Demonstrated Effects | Effector Molecules/Pathways | Expression Changes |
|---|---|---|---|
| Prostate Cancer | Anti-inflammatory effects | PG/LTs | Downregulation [126] |
| Breast Cancer | Anti-inflammatory effects, inhibition of metastasis | COX2/PGE2/Twist1 | Downregulation [126] |
| Hepatocellular Carcinoma | Inhibition of proliferation | Farnesoid X receptor | Upregulation [126] |
| Colorectal Cancer | Induction of apoptosis | Caspase 3 | Activation [126] |
| Pancreatic Cancer | Anti-inflammatory effects | IL-1β/IL-6 | Downregulation [126] |
| Nasopharyngeal Carcinoma | Protective metabolic pathway | Alpha-linolenic acid metabolism | Protective factor [128] |
The protective role of alpha-linolenic acid metabolism has also been demonstrated in nasopharyngeal carcinoma (NPC), where bioinformatics analysis of TCGA data identified it as a protective metabolic pathway [128]. A prognostic model based on alpha-linolenic acid metabolism-related genes (DEFB4B, FOXL2NB, MDGA2, RTL1, SLURP2, TMEM151B, and TSPAN19) effectively stratified NPC patients into distinct risk categories, with the low-risk group showing enhanced sensitivity to immunotherapy and lapatinib treatment [128].
Alpha-Lipoic Acid (ALA): Anticancer Evidence and Mechanisms
Pro-Oxidant and Redox-Modulating Activities
Unlike its fatty acid counterpart, alpha-lipoic acid exhibits a dual nature in redox biology, functioning as both an antioxidant in normal cells and a pro-oxidant in cancer cells. This contextual activity makes it particularly interesting for oncological applications. In its reduced form as dihydrolipoic acid (DHLA), it creates a potent redox couple with unique properties [127].
In cancer cells, alpha-lipoic acid induces oxidative stress by increasing reactive oxygen species (ROS) levels, leading to pro-apoptotic signaling. This pro-oxidant effect is particularly pronounced in certain cancer types, including prostate cancer, where ALA treatment significantly increased ROS levels and HIF-1α expression, activating the JNK/caspase-3 signaling pathway and resulting in apoptosis [129].
Molecular Pathways and Signaling Modulation
Alpha-lipoic acid influences multiple oncogenic signaling pathways through complex mechanisms:
AMPK-p53 Axis Activation: In hepatocellular carcinoma, ALA activates AMPK, which in turn inhibits mTOR signaling and promotes p53 activation, leading to cell cycle arrest, increased apoptosis, and reduced metastatic potential [130]. This AMPK-p53 axis modulation represents a promising metabolic approach to HCC treatment.
HIF-1α/JNK/Caspase-3 Pathway: In advanced prostate cancer cells (22Rv1 and C4-2B), ALA supplementation elevated ROS levels, increased HIF-1α expression, and activated the JNK/caspase-3 signaling pathway, resulting in cell cycle arrest in S-phase and apoptosis [129].
EGFR Downregulation: In human lung cancer cells, ALA inhibited proliferation through Grb2-mediated EGFR downregulation, affecting a key driver of oncogenic signaling [131].
Integrin Modulation and Anoikis Sensitization: ALA sensitized lung cancer cells to chemotherapeutic agents and anoikis via integrin β1/β3 downregulation, potentially limiting metastatic potential [131].
The following diagram illustrates the key molecular pathways modulated by alpha-lipoic acid in cancer cells:
Cancer-Type Specific Evidence
Prostate Cancer
In advanced human prostate cancer cell lines (22Rv1 and C4-2B), alpha-lipoic acid demonstrated significant anti-tumor effects in a dose-dependent manner [129]. Treatment with 500 μM ALA for 48 hours reduced cell viability by approximately 60-70% in both MTT and colony formation assays. The compound also significantly impaired migratory and invasive capacity in wound healing and Transwell migration assays [129].
A particularly relevant finding for prostate cancer management was ALA's effect on bone cell differentiation. Prostate cancer frequently metastasizes to bone, causing significant morbidity. ALA supplementation diminished PCa-mediated differentiation of both osteoblasts and osteoclasts in MC3T3-E1 and bone marrow macrophages (BMMs), suggesting potential for mitigating bone metastasis complications [129].
Hepatocellular Carcinoma
In HCC, alpha-lipoic acid has shown promise through modulation of the AMPK-p53 axis [130]. Preclinical studies have demonstrated ALA's efficacy in reducing tumor growth and metastasis in HCC models, suggesting its potential as an adjunct therapy. The compound inhibits epithelial-mesenchymal transition (EMT), a critical process in cancer metastasis, through this pathway [130].
Other Cancers
Alpha-lipoic acid has demonstrated anticancer activity across diverse cancer types:
- Breast, Ovarian, Colorectal, and Lung Cancer: ALA alone decreased cell viability and proliferation in these cancer cell lines and showed synergistic effects with chemotherapy [131].
- Thyroid Cancer: ALA decreased cell migration and invasion in thyroid cancer cell lines, potentially limiting metastatic behavior [131].
- Glioblastoma: The FASN-inhibitor TVB-2640 (which shares some metabolic targets with ALA) combined with bevacizumab showed favorable antitumor activity in a phase II clinical trial [132].
Experimental Models and Methodologies
Standardized Experimental Protocols
To facilitate reproducibility and comparative analysis across studies, we have compiled key methodological details from seminal research on both ALA compounds:
In Vitro Assessment of Anti-Proliferative Effects
- Cell Viability Assay (MTT): Seed 2500 cells/well in 96-well plates overnight in complete medium. Treat with ALA at concentrations ranging from 50-2000 μM for 48 hours. Add MTT reagent and incubate for 2 hours at 37°C. Dissolve formazan crystals in DMSO and measure absorbance at 570 nm [129].
- Colony Formation Assay: Seed 1000 cells/well in 6-well plates. After 24 hours, treat with 500 μM ALA. Refresh medium every 48 hours for 14 days. Fix with 0.5% crystal violet, count colonies using Image-J [129].
Migration and Invasion Assessment
- Wound Healing Assay: Seed 1×10ⶠcells in 35 mm culture dishes. Treat with 500 μM ALA for 48 hours. Create scratch with sterile pipette tip and monitor migration at 0, 24, and 48 hours using inverted microscopy [129].
- Transwell Invasion Assay: Seed treated viable cells in serum-free medium in Matrigel-coated Boyden chambers. Place 20% FBS medium in lower chamber as chemoattractant. After 48 hours, stain and fix invaded cells with Diff-Quick stain, count cells across multiple fields [129].
Molecular Mechanism Elucidation
- Western Blot Analysis: Prepare cell lysates in RIPA buffer with protease/phosphatase inhibitors. Resolve 20-40 μg protein by SDS-PAGE, transfer to PVDF membrane. Block with 5% non-fat milk, incubate with primary antibodies overnight at 4°C, followed by HRP-conjugated secondary antibodies for 1 hour. Detect using ECL reagent [129].
- ROS Measurement: Use DCFDA cellular ROS detection assay kit. Incubate cells with 25 μM DCFDA for 30 minutes at 37°C. Analyze fluorescence intensity using flow cytometry or fluorescence microscopy [129].
Research Reagent Solutions
Table 3: Essential Research Reagents for Investigating ALA Mechanisms in Cancer Models
| Reagent/Category | Specific Examples | Research Application | Key Functions |
|---|---|---|---|
| Cell Culture Models | 22Rv1, C4-2B (PCa); MC3T3-E1 (osteoblast); BMMs (osteoclast) | In vitro mechanism studies | Represent advanced prostate cancer, bone microenvironment |
| Antibodies | Anti-HIF-1α, anti-phospho-JNK, anti-caspase-3, anti-cleaved caspase-3 | Protein expression analysis | Detect pathway activation in Western blot |
| Chemical Inhibitors | SP600125 (JNK inhibitor), NAC (ROS scavenger) | Pathway validation | Confirm mechanism of action |
| Detection Assays | MTT, colony formation, DCFDA, Annexin V/PI | Phenotypic characterization | Assess viability, proliferation, ROS, apoptosis |
| Animal Models | Mouse xenograft models | In vivo validation | Evaluate efficacy, toxicity, pharmacokinetics |
Therapeutic Applications and Clinical Translation
Combination Therapy Strategies
Both ALA compounds show enhanced efficacy when combined with conventional therapies:
Alpha-Lipoic Acid Clinical Combinations
- Pancreatic Cancer: Intravenous ALA (300-600 mg twice weekly) plus oral low-dose naltrexone (4.5 mg once daily) produced complete responses in case reports of patients with pancreatic cancer [131].
- Supportive Care: ALA combined with Boswellia Serrata, methylsulfonylmethane, and bromelain reduced pain and sensorimotor impairment in chemotherapy-induced peripheral neuropathy [131].
- Metabolic Modulation: The combination of ALA with hydroxycitrate enhanced efficacy against tumor development in experimental models and case reports [131].
Alpha-Linolenic Acid Combination Potential
- Renal Cell Carcinoma: ALA combined with PPAR-γ agonist rosiglitazone and COX-2 inhibitor N-(3-pyridyl) indomethacinamide showed enhanced anti-proliferative effects [126].
- Immunotherapy Enhancement: Bioinformatics analysis suggests ALA metabolism influences tumor immune microenvironment, potentially enhancing response to immunotherapy [128].
Diagnostic and Prognostic Applications
Beyond therapeutic applications, ALA metabolism has demonstrated value in cancer prognosis:
- In nasopharyngeal carcinoma, a 7-gene prognostic signature based on alpha-linolenic acid metabolism-related genes effectively stratified patients into distinct risk categories with differential sensitivity to treatments [128].
- For alpha-linolenic acid, biomarker levels in blood provided superior prognostic value compared to dietary intake assessments for colorectal cancer risk prediction [122].
The following diagram summarizes the experimental workflow for evaluating ALA compounds in cancer models:
The evidence from cancer studies reveals that both alpha-linolenic acid and alpha-lipoic acid offer unique therapeutic opportunities in oncology, though through fundamentally distinct mechanisms. Alpha-linolenic acid, as an essential fatty acid, demonstrates multimodal anticancer activity including anti-proliferative, pro-apoptotic, anti-inflammatory, and anti-metastatic effects across various cancer types, with particularly strong epidemiological support for colorectal cancer risk reduction when measured via blood biomarkers. Alpha-lipoic acid, functioning as a redox modulator, exhibits context-dependent pro-oxidant activity in cancer cells, inducing apoptosis through multiple pathways including ROS/HIF-1α/JNK/caspase-3 signaling in prostate cancer and AMPK-p53 axis modulation in hepatocellular carcinoma.
Future research should prioritize several key areas: (1) standardization of ALA compound terminology to prevent confusion in scientific literature; (2) validation of biomarker-driven approaches for patient stratification; (3) exploration of synergistic combinations with conventional therapies and immunotherapy; and (4) conduct of well-designed clinical trials that incorporate mechanistic biomarkers to validate preclinical findings. Both ALA compounds represent promising avenues for cancer prevention and treatment that warrant further investigation through collaborative multidisciplinary approaches.
Alpha-linolenic acid (ALA), a plant-derived omega-3 fatty acid, presents a compelling case for sustainable nutritional sourcing amid growing global demand for long-chain polyunsaturated fatty acids (LC-PUFA). This technical review examines ALA's economic and ecological positioning relative to marine-derived eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). While ALA demonstrates significant cardioprotective benefits and lower production costs, its limited conversion efficiency to EPA and DHA in humans remains a critical metabolic consideration. Emerging biotechnological approaches using microbial and algal systems offer promising pathways to overcome current supply chain limitations and bioavailability challenges, potentially bridging the gap between sustainability and therapeutic efficacy for research and drug development applications.
Omega-3 fatty acids constitute a family of polyunsaturated fats characterized by the presence of a double bond three atoms away from the terminal methyl group in their chemical structure. The primary omega-3 fatty acids include alpha-linolenic acid (ALA, C18:3), eicosapentaenoic acid (EPA, C20:5), and docosahexaenoic acid (DHA, C22:6). ALA is considered an essential fatty acid because humans cannot synthesize it endogenously due to the lack of delta-12 desaturase (D12Des), necessitating dietary intake [133].
The metabolic pathway for omega-3 biosynthesis begins with ALA, which undergoes a series of elongation and desaturation reactions to form EPA and eventually DHA. This conversion occurs through the action of enzymes including delta-6-desaturase, elongase-5, and delta-5-desaturase. However, this biosynthetic pathway operates inefficiently in humans, with conversion rates estimated at only 0.2-0.8% for ALA to EPA and less than 4% for ALA to DHA in men, and slightly higher at 21% and 9% respectively in women [133]. This limited conversion efficiency significantly impacts the relative bioefficacy of ALA compared to pre-formed EPA and DHA.
The metabolic relationships and conversion pathways between ALA and long-chain omega-3s are illustrated below:
Figure 1: ALA Metabolic Pathway and Conversion Limitations
Health Benefits and Research Evidence for ALA
Cardioprotective and Mortality Effects
Substantial clinical evidence supports the health benefits of ALA consumption. A comprehensive systematic review and meta-analysis of 41 prospective cohort studies totaling 1,197,564 participants demonstrated that higher ALA intake is significantly associated with reduced risk of mortality from all causes (pooled relative risk 0.90), cardiovascular disease (0.92), and coronary heart disease (0.89) [134]. The dose-response analysis revealed that a 1 g/day increase in ALA intake (equivalent to approximately one tablespoon of canola oil or 0.5 ounces of walnut) was associated with a 5% lower risk of all-cause and cardiovascular mortality [134].
Notably, tissue biomarkers of ALA showed a significant inverse association with all-cause mortality (pooled RR 0.95), supporting the biological plausibility of ALA's cardioprotective effects beyond mere association [134]. However, the same analysis indicated a slightly higher risk of cancer mortality (RR 1.06) with high ALA intake, suggesting potential differential effects across disease pathologies that warrant further investigation.
Comparative Bioefficacy and Health Outcomes
While marine-derived EPA and DHA have demonstrated robust cardioprotective effects in numerous clinical trials, the evidence for ALA suggests more modest benefits. The American Heart Association recommends 4 g/day of EPA and DHA or EPA only as daily supplements for cardiovascular protection [133], whereas no equivalent official recommended dose exists specifically for ALA, though the Food and Agriculture Organization (FAO) of the United Nations and WHO recommend a daily intake of 250 mg of EPA and DHA per adult [135].
The health benefits observed for ALA have been partially attributed to its precursor role in converting to EPA in the body [49]. However, the inefficient conversion means that achieving equivalent tissue levels of EPA and DHA from ALA alone would require substantially higher dietary intake. This metabolic limitation has important implications for researchers developing therapeutic formulations and nutritional interventions targeting specific tissue concentrations of long-chain omega-3s.
Market Dynamics and Production Costs
The global omega-3 market has experienced significant growth, valued at USD 2.49 billion in 2019 and expected to expand at a compound annual growth rate (CAGR) of 7% through 2027 [135]. More recent estimates indicate the market was USD 2.10 billion in 2020 and is predicted to reach USD 3.61 billion by 2028 [133]. The algae omega-3 segment specifically reached US$ 1.2 billion in 2022 and is expected to reach US$ 1.7 billion by 2030, growing with a CAGR of 4.5% [136].
Traditional fish oil remains the primary source of omega-3 fatty acids in the market, with an average wholesale price of approximately $14/kg [133]. However, this price fluctuates based on fishing yields, environmental factors, and purification costs. Plant-derived ALA sources such as flaxseed, walnuts, and canola oil offer significant economic advantages, with production costs typically 30-50% lower than refined fish oil equivalents due to established agricultural infrastructure and simpler extraction processes.
Table 1: Comparative Economic Analysis of Omega-3 Sources
| Source Type | Representative Sources | Primary Omega-3 Form | Estimated Production Cost (USD/kg) | Market Share (2022) | Projected Growth Rate |
|---|---|---|---|---|---|
| Plant-Based | Flaxseed, Chia, Canola oil | ALA | $6-10 | 5-10% [136] | Moderate (3-5% CAGR) |
| Marine Fish Oil | Sardine, Anchovy | EPA/DHA | $14-18 [133] | 60-70% | Constrained (2-4% CAGR) |
| Algal Oil | Schizochytrium, Ulkenia | DHA/EPA | $20-35 | 15-20% [136] | High (7-9% CAGR) |
| Engineered Microbial | Yarrowia lipolytica | EPA | $12-25 (projected) [133] | <5% | Very High (>10% CAGR) |
Production Scalability and Infrastructure Requirements
Plant-derived ALA production benefits from established global agricultural systems capable of rapid scaling with relatively low capital investment. Flaxseed production, for example, yields approximately 800-1200 kg of oil per hectare with ALA content of 50-55% [49]. This compares favorably to fish oil production, which requires extensive fishing operations, processing facilities, and is constrained by natural fishery limits and seasonal variations.
Algal production systems represent an intermediate position, requiring significant bioreactor infrastructure but offering higher potential yields per unit area than terrestrial agriculture. Closed-loop photobioreactor systems can produce algal biomass with 20-30% DHA content year-round, independent of seasonal variations [136]. However, the high capital and operational costs of these systems currently limit their economic competitiveness with plant-based ALA sources.
Sustainability Assessment
Environmental Impact Metrics
The sustainability advantages of plant-derived ALA over marine-sourced omega-3s are substantial. Marine-derived omega-3 production faces critical sustainability challenges including overfishing, bycatch issues, ecosystem disruption, and uncertainty in stock management [137]. Current fishing practices are insufficient to meet global demand for omega-3 fatty acids without further depleting marine resources [135].
Table 2: Comparative Environmental Impact Assessment
| Parameter | Plant-Derived ALA | Marine Fish Oil | Algal Omega-3 |
|---|---|---|---|
| Land/Water Use | Moderate agricultural land use; seasonal water requirements | Minimal land use but significant marine ecosystem impact | Limited physical footprint; controlled water recycling |
| Carbon Footprint | Low to moderate (0.5-2 kg COâ‚‚eq/kg oil) | Moderate to high (2-5 kg COâ‚‚eq/kg oil) from fishing vessels | Variable (1-4 kg COâ‚‚eq/kg oil) depending on energy source |
| Ecosystem Impact | Conventional agriculture impacts (pesticides, fertilizers) | Overfishing, bycatch, marine food web disruption [135] | Minimal wild ecosystem impact when using closed systems |
| Resource Depletion | Renewable through crop rotation | Non-renewable at current extraction rates [133] | Highly renewable with proper nutrient management |
| Production Yield | 800-1200 kg oil/hectare (flaxseed) | Declining yields from wild fisheries | 5000-10,000 kg oil/hectare (projected) [136] |
Supply Chain Resilience and Geographic Considerations
Plant-based ALA sources offer superior supply chain resilience due to distributed production across multiple geographic regions and climate zones. Flaxseed cultivation occurs across North America, Europe, and Asia, providing natural diversification against regional crop failures or climate disruptions. In contrast, marine sources are concentrated in specific fishing grounds (Peru, Chile, Norway) creating vulnerability to environmental fluctuations, political regulations, and transportation disruptions.
Algal production systems can be geographically distributed but remain concentrated in regions with technical expertise and favorable economic conditions. Recent developments in heterotrophic microalgae cultivation using alternative carbon sources like volatile fatty acids recovered from waste streams promise improved sustainability profiles and reduced production costs [135]. These innovations could further enhance the competitive position of algal omega-3s relative to both plant ALA and marine sources.
Technical and Experimental Considerations for Research Applications
Research Reagent Solutions for Omega-3 Studies
Table 3: Essential Research Materials for Omega-3 Investigations
| Reagent/Material | Function/Application | Technical Considerations |
|---|---|---|
| Gas Chromatography-Mass Spectrometry (GC-MS) | Fatty acid quantification and profiling | Requires fatty acid methylation; critical for accurate EPA/DHA measurement in conversion studies |
| Cell Culture Models (HepG2, Caco-2) | Intestinal absorption and hepatic metabolism studies | Enables investigation of ALA conversion efficiency and transport mechanisms |
| Δ-6-Desaturase Antibodies | Protein expression analysis in metabolic studies | Essential for monitoring key enzymatic step in ALA to EPA conversion pathway |
| Stable Isotope-Labeled ALA (¹³C) | Metabolic tracing studies | Allows precise tracking of ALA conversion to EPA/DHA in vitro and in vivo |
| Lipid Extraction Solvents (Chloroform-Methanol) | Lipid extraction from tissues and biological samples | Standard 2:1 chloroform:methanol ratio for Folch method; critical for reproducible yields |
| Omega-3 Enriched Media Formulations | Cell culture studies of omega-3 effects | Must maintain antioxidant protection against lipid peroxidation during experiments |
Methodological Framework for ALA Bioefficacy Studies
The experimental workflow for investigating ALA metabolism and comparative efficacy requires careful methodological planning. The following diagram illustrates a standardized approach for preclinical assessment of ALA bioavailability and conversion efficiency:
Figure 2: Experimental Workflow for ALA Bioefficacy Studies
For clinical investigations, particularly those examining the cardioprotective effects of ALA, the following protocol details ensure methodological rigor:
- Participant Selection: Recruit subjects with controlled background diets and exclude those taking omega-3 supplements or medications affecting lipid metabolism
- Intervention Design: Implement randomized, double-blind, placebo-controlled trial with parallel groups receiving either:
- ALA-rich oil (flaxseed, canola) providing 2-4 g/day ALA
- EPA+DHA preparation (fish/algal oil) providing 500-1000 mg/day
- Placebo oil (olive/safflower oil with low omega-3 content)
- Duration: Minimum 8-12 weeks intervention period to assess tissue incorporation
- Outcome Measures:
- Primary: Plasma phospholipid fatty acid composition
- Secondary: Inflammatory markers (CRP, IL-6, TNF-α), lipid profiles, blood pressure
- Compliance Monitoring: Capsule counts plus plasma fatty acid analysis to verify adherence
Future Perspectives and Research Directions
Technological Innovations in Omega-3 Production
Biotechnological advances present promising pathways for overcoming current limitations in omega-3 production. Metabolic engineering of yeast strains such as Yarrowia lipolytica demonstrates potential for sustainable EPA production through fermentation technology [133]. Similarly, advances in microalgae cultivation using heterotrophic systems and waste-derived carbon sources offer opportunities for reducing production costs while maintaining sustainability credentials [135].
The conversion yield from substrates to omega-3 fatty acids remains a major challenge for microbial fermentation systems. Current research focuses on optimizing fermentation conditions, engineering improved enzyme kinetics in the omega-3 biosynthetic pathway, and developing cost-effective purification methods. Success in these areas could position microbial production as a competitive alternative to both plant ALA and marine-sourced omega-3s.
Emerging Applications and Market Opportunities
The aging global population and increasing focus on preventive healthcare are driving expanded applications for omega-3 fatty acids. Beyond cardiovascular health, research continues to explore potential benefits for neuropsychiatric disorders, cognitive function, immune support, and healthy aging [137] [135]. Plant-derived ALA offers particular promise for vegetarian and vegan formulations, representing a growing segment of the nutritional products market.
Geographic market expansion presents additional opportunities, particularly in Asia-Pacific regions where increasing disposable income and health consciousness are driving demand for omega-3 products [136]. The alignment of ALA sources with plant-based dietary trends positions this omega-3 source for continued growth, though education about conversion limitations remains necessary for appropriate consumer expectations.
Plant-derived alpha-linolenic acid presents a economically competitive and environmentally sustainable source of omega-3 fatty acids, though with recognized limitations in bioconversion to long-chain EPA and DHA. For researchers and product developers, ALA offers distinct advantages in supply chain stability, production cost, and environmental impact compared to marine sources. Emerging biotechnological approaches hold promise for bridging the efficacy gap between ALA and marine omega-3s through improved conversion efficiency and alternative production systems. Future research should focus on optimizing ALA bioavailability, understanding factors influencing conversion efficiency, and developing innovative delivery systems to maximize its health benefits while leveraging its sustainability advantages.
Risk-Benefit Analysis of ALA Supplementation
Alpha-lipoic acid (ALA), also known as thioctic acid, is a potent endogenous mitochondrial compound that functions as an essential cofactor in energy metabolism. This whitepaper presents a systematic risk-benefit analysis of ALA supplementation, framed within the broader context of alpha-lipoic acid metabolism and health benefits research. Unlike alpha-linolenic acid (an omega-3 fatty acid also abbreviated ALA), alpha-lipoic acid is an organosulfur compound characterized by a unique amphiphilic structure that confers both water and fat solubility [1] [138]. This biochemical property enables ALA to function throughout the body, including passage across the blood-brain barrier, distinguishing it from many other antioxidants [1]. The biological activity of ALA is primarily attributed to the redox couple formed between its oxidized (ALA) and reduced (dihydrolipoic acid, DHLA) forms, which creates one of nature's most potent antioxidant systems [1]. This analysis examines the therapeutic potential, molecular mechanisms, and safety profile of ALA supplementation based on current clinical evidence and mechanistic studies, providing researchers and drug development professionals with a comprehensive assessment of its risk-benefit ratio.
Biochemical Properties and Molecular Mechanisms
Unique Antioxidant Properties
The ALA/DHLA redox couple represents an exceptional biological antioxidant system with a standard reduction potential of -0.32 V [1]. This system exhibits multifunctional antioxidant capacity through several distinct mechanisms:
- Direct free radical quenching: DHLA effectively scavenges peroxyl radicals in both membrane and aqueous phases without requiring glutathione or α-tocopherol [1]
- Metal chelation capacity: Both ALA and DHLA chelate redox-active metals due to adjacent sulfur atoms and carboxyl groups, with DHLA chelating Fe³âº, Co²âº, Ni²âº, Cd²âº, Cu²âº, Zn²âº, Pb²âº, and Hg²âº, while ALA preferentially binds Mn²âº, Cu²âº, Zn²âº, and Pb²⺠[1]
- Antioxidant regeneration: DHLA reduces ascorbyl radicals produced during ascorbate oxidation and regenerates other antioxidants including vitamins C and E [1] [138]
- Glutathione enhancement: ALA acts as a transcriptional inducer of genes regulating glutathione synthesis and increases substrate availability, thereby boosting intracellular glutathione levels by up to 30%-70% [1] [138]
The following diagram illustrates the multifaceted antioxidant mechanisms of ALA:
Anti-inflammatory and Signaling Pathways
Beyond its antioxidant functions, ALA demonstrates significant anti-inflammatory activity through modulation of critical signaling pathways:
- NF-κB inhibition: ALA suppresses nuclear factor kappa B (NF-κB), a master regulator of proinflammatory genes encoding cytokines, adhesion molecules, and chemokines [1]
- Adhesion molecule downregulation: ALA prevents TNF-α-induced upregulation of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) in endothelial cells [1]
- Nitric oxide modulation: ALA increases vasodilation through nitric oxide mediation, improving endothelial function [139]
- AMPK regulation: ALA suppresses AMP-activated protein kinase (AMPK), which plays a role in appetite regulation and may contribute to metabolic effects [140]
Quantitative Analysis of Clinical Efficacy
Cardiometabolic Risk Factors
A 2025 systematic review and dose-response meta-analysis of 63 randomized controlled trials (RCTs) demonstrated that ALA supplementation significantly improves multiple cardiometabolic risk factors [141]:
Table 1: Effects of ALA Supplementation on Cardiometabolic Parameters
| Parameter | Weighted Mean Difference | 95% Confidence Interval | P-value |
|---|---|---|---|
| Homeostatic model assessment for insulin resistance | -0.74 | -1.17, -0.31 | 0.001 |
| Fasting blood glucose (mg/dL) | -5.28 | -7.21, -3.35 | <0.001 |
| Hemoglobin A1c (%) | -0.40 | -0.66, -0.14 | 0.003 |
| Fasting insulin (mU/mL) | -1.70 | -2.88, -0.53 | 0.004 |
| Body weight (kg) | -0.64 | -1.04, -0.24 | 0.002 |
| Body mass index (kg/m²) | -0.27 | -0.44, -0.10 | 0.002 |
| Waist circumference (cm) | -1.10 | -1.66, -0.54 | <0.001 |
| Fat mass (kg) | -1.42 | -2.53, -0.31 | 0.012 |
| Total cholesterol (mg/dL) | -3.91 | -7.35, -0.46 | 0.026 |
| Triglycerides (mg/dL) | -2.90 | -5.21, -0.59 | 0.014 |
Notably, the same meta-analysis found no significant effects on LDL cholesterol, HDL cholesterol, or blood pressure, suggesting a selective impact on specific metabolic pathways [141].
Blood Pressure Effects
A separate 2023 systematic review and meta-analysis of 11 RCTs with 674 participants specifically evaluated ALA's effects on blood pressure, demonstrating significant reductions in both systolic and diastolic blood pressure [139]:
Table 2: Blood Pressure Outcomes from ALA Supplementation
| Parameter | Weighted Mean Difference | 95% Confidence Interval | P-value | Effective Dosage |
|---|---|---|---|---|
| Systolic Blood Pressure | -5.46 mmHg | -9.27, -1.65 | <0.001 | <800 mg/day for ≤12 weeks |
| Diastolic Blood Pressure | -3.36 mmHg | -4.99, -1.74 | <0.001 | <800 mg/day for ≤12 weeks |
The analysis revealed that ALA administration significantly reduced both systolic and diastolic blood pressure at dosages below 800 mg/day when administered for 12 weeks or less, suggesting that moderate-term intervention at lower doses may be optimal for blood pressure management [139].
Dose-Response Relationships
The efficacy of ALA supplementation demonstrates dose-dependent characteristics across different health outcomes:
- Diabetic neuropathy: 300-600 mg/day intravenously or 600-1,800 mg orally demonstrated efficacy for diabetic neuropathy symptoms [140] [138]
- Weight management: 1,200 mg/day for eight weeks induced mild weight loss with reduced waist circumference [138]
- Blood pressure: Doses below 800 mg/day for ≤12 weeks showed significant antihypertensive effects [139]
- Antioxidant protection: Doses of 300-1,200 mg help increase glutathione levels and enhance antioxidant capacity [138]
Therapeutic Applications and Clinical Evidence
Diabetes and Metabolic Syndrome
ALA supplementation shows particular promise in managing diabetes and metabolic disorders through multiple mechanisms:
- Insulin sensitivity enhancement: ALA improves insulin sensitivity by modulating insulin signaling pathways and reducing oxidative stress in insulin-responsive tissues [141] [138]
- Diabetic neuropathy management: Intravenous ALA (300-600 mg/day) significantly improves symptoms of diabetic neuropathy, including pain, burning, tingling, and numbness [140] [138]
- Glycemic control: Multiple RCTs demonstrate ALA's ability to reduce fasting blood glucose, hemoglobin A1c, and fasting insulin levels [141]
- Cardiovascular autonomic neuropathy risk reduction: ALA supplementation may lower the risk of CAN, a serious diabetic complication affecting 25% of diabetes patients and associated with increased mortality [138]
Neurological Protection
The amphiphilic nature of ALA enables penetration across the blood-brain barrier, supporting its potential neuroprotective applications:
- Cognitive decline mitigation: ALA can protect neurons from oxidative damage and may help prevent age-related cognitive decline, though human studies are less established than preclinical data [138]
- Neurodegenerative disease potential: Research indicates ALA's ability to protect against protein aggregation and mitochondrial dysfunction in neurodegenerative models [1]
- Peripheral neuropathy: Strongest evidence exists for diabetic neuropathy, with potential applications to other forms of nerve damage [140] [138]
Additional Therapeutic Applications
Emerging research suggests potential benefits in several other health domains:
- Skin health: Topical creams containing 5% ALA demonstrated efficacy in improving epidermal thickness and reducing fine lines in placebo-controlled trials [138]
- Vision protection: ALA may protect against retinal damage and vision loss associated with oxidative stress, particularly in diabetic retinopathy and age-related eye diseases [138]
- Weight management: Meta-analyses indicate modest weight loss benefits (1.5-5 pounds) with ALA supplementation, though effects on waist circumference are less consistent [140] [138]
Safety Profile and Risk Assessment
Adverse Effects and Tolerability
ALA supplementation is generally well-tolerated, with a favorable safety profile at recommended dosages:
- Common side effects: Minor adverse effects include insomnia, fatigue, diarrhea, skin rash, and gastrointestinal discomfort [138]
- Dosage considerations: Doses from 200-2,400 mg daily are considered safe, with higher doses offering no additional benefits and potentially increasing side effect risk [140]
- Long-term safety: Limited data exist on long-term ALA use, though short-to-medium-term studies (up to 4 years) demonstrate good tolerability [142]
Special Population Considerations
Specific precautions apply to particular patient populations:
- Diabetes patients: ALA may enhance glucose-lowering medications, potentially increasing hypoglycemia risk; blood glucose monitoring and medication adjustment may be necessary [140]
- Thyroid conditions: ALA may reduce thyroid hormone levels, requiring monitoring in patients with thyroid disorders [140]
- Pregnancy and lactation: ALA supplements are not recommended during pregnancy or breastfeeding due to insufficient safety data [140]
- Pediatric populations: Safety and efficacy have not been established in children [140]
- Cancer patients: Potential interactions with certain chemotherapeutic agents require careful evaluation [140]
Drug and Nutrient Interactions
ALA may interact with several medications and nutrients:
- Antidiabetic drugs: Potentiates effects of insulin and oral hypoglycemic agents [140]
- Thyroid medications: May interfere with thyroid hormone replacement therapy [140]
- Chemotherapeutic agents: Potential interactions with certain cancer treatments [140]
- Anticoagulants: May enhance effects of blood-thinning medications [140]
Experimental Protocols and Research Methodology
Standardized Clinical Trial Protocols
For researchers investigating ALA supplementation, the following experimental protocols represent methodologies from high-quality clinical trials:
Protocol 1: Cardiometabolic Parameters Study
- Design: Randomized, double-blind, placebo-controlled trial
- Participants: Adults with metabolic syndrome or type 2 diabetes
- Intervention: 300-600 mg/day ALA (R-form preferred for bioavailability)
- Duration: 8-16 weeks
- Primary endpoints: HOMA-IR, fasting glucose, HbA1c, lipid profile
- Secondary endpoints: Body composition, inflammatory markers, blood pressure
- Methodology: Standardized dosing 30 minutes before meals, consistent timing of assessments [141] [142]
Protocol 2: Neuropathy Symptom Assessment
- Design: Randomized, double-blind, placebo-controlled trial
- Participants: Diabetic patients with confirmed peripheral neuropathy
- Intervention: 600 mg/day ALA intravenously (3-week acute phase) or 600-1,800 mg orally (maintenance)
- Duration: 3 weeks (IV) or 3-6 months (oral)
- Assessment tools: Total Symptom Score (TSS), Neuropathy Impairment Score (NIS), nerve conduction studies
- Endpoint evaluation: Weekly symptom assessments, baseline and final neurological examinations [140] [138]
Laboratory Assessment Methods
Comprehensive evaluation of ALA's effects should include these key laboratory assessments:
- Oxidative stress markers: Plasma glutathione (GSH/GSSG ratio), malondialdehyde (MDA), 8-isoprostane, protein carbonyl content
- Inflammatory biomarkers: High-sensitivity C-reactive protein (hs-CRP), TNF-α, IL-6, NF-κB activity
- Metabolic parameters: Oral glucose tolerance test, hyperinsulinemic-euglycemic clamp for insulin sensitivity, continuous glucose monitoring
- Mitochondrial function: Respiratory chain enzyme activities, ATP production, mitochondrial membrane potential
Research Reagent Solutions
Table 3: Essential Research Reagents for ALA Investigations
| Reagent/Category | Specific Examples | Research Application |
|---|---|---|
| ALA Formulations | R-ALA enantiomer, Na-R-ALA, racemic ALA | Bioavailability studies, dose-response investigations |
| Antioxidant Assays | DCFH-DA, ORAC assay, TEAC assay | Quantification of antioxidant capacity |
| Molecular Biology Kits | NF-κB activation assay, Nrf2 ELISA, glutathione assay kits | Mechanism of action studies |
| Metabolic Assays | Glucose uptake assays, insulin signaling phospho-arrays, adipocyte differentiation kits | Metabolic pathway analysis |
| Analytical Standards | Deuterated ALA, DHLA standards, lipid peroxidation standards | LC-MS/MS method development and validation |
The risk-benefit analysis of ALA supplementation demonstrates a favorable profile for specific clinical applications, particularly in diabetes management, cardiometabolic health, and neuropathy treatment. The most robust evidence supports using ALA at doses of 300-600 mg/day for diabetic neuropathy and 600-800 mg/day for metabolic parameters, with treatment durations of 8-16 weeks showing optimal efficacy. The mechanisms involve multifaceted antioxidant and anti-inflammatory effects, with the ALA/DHLA redox couple serving as a central component. Future research should focus on long-term safety assessment, molecular mechanisms of action, and potential applications in neurodegenerative diseases. For drug development professionals, ALA represents a promising candidate for combination therapies and novel formulations aimed at enhancing bioavailability and tissue-specific targeting.
Future Directions for Clinical Translation and Drug Development
The process of translating basic scientific research into clinically effective therapies represents one of the most significant challenges in modern medicine. Translational research forms a critical bridge between basic scientific inquiry and clinical application, playing an indispensable role in converting laboratory discoveries into treatments that directly benefit patients [143]. This "bench-to-bedside" process encompasses the identification of therapeutic targets, development of candidate drugs, preclinical testing in animal models, and eventual evaluation in human clinical trials [144]. Despite substantial investments in basic science and technological advancements, the translation of these findings into therapeutic advances has proceeded far more slowly than anticipated, with a significant majority of research projects failing before they ever reach human testing [143].
The crisis involving the translatability of preclinical science to human applications is widely recognized across both academic and industrial sectors [143]. Current data reveals a stark reality: the process of developing a new drug, from initial testing to final regulatory approval, typically spans more than 13 years, with approximately 95% of drugs entering human trials ultimately failing [143]. This high attrition rate contributes to staggering development costs, averaging $2.6 billion per newly approved drug [143]. These challenges are particularly relevant in the context of nutritional biochemistry and phytochemical research, where the pathway from identifying health benefits of natural compounds like alpha-linolenic acid (ALA) to developing targeted therapies involves navigating complex metabolic pathways, bioavailability issues, and demonstrating clear clinical efficacy.
Current Challenges in Clinical Translation
The "Valley of Death" in Translational Research
The significant gap between basic research discoveries and their clinical application has been termed the "Valley of Death" in translational research [143]. This metaphor describes the funding and support abyss where promising basic science findings frequently languish due to insufficient resources, expertise, or infrastructure to advance them toward therapeutic development. Several interconnected factors contribute to this challenge:
- High attrition rates: Approximately 80-90% of research projects fail before reaching human testing, with only 0.1% of new drug candidates progressing from preclinical research to approved drugs [143]
- Predictability limitations: Traditional animal models often fail to accurately predict human responses, with lack of effectiveness and poor safety profiles being primary causes of failure despite promising preclinical results [143]
- Resource intensiveness: The lengthy timeline (exceeding 13 years) and enormous financial investment ($2.6 billion per approved drug) create significant barriers for translation [143]
Specific Challenges in ALA Research and Development
Research into alpha-linolenic acid and its therapeutic applications faces several specific hurdles that complicate the translational pathway:
- Metabolic complexity: ALA serves as a precursor to longer-chain omega-3 fatty acids EPA and DHA, but conversion rates are highly variable among individuals [145]. Estimates of ALA conversion to EPA range from 0.2% to 21%, while conversion to DHA is even more limited (0.05%-9%), with higher rates typically observed in women [145]
- Bioavailability and stability: ALA is highly susceptible to oxidation due to its three double bonds, creating formulation challenges for pharmaceutical development [146]
- Competitive metabolism: The desaturation and elongation of ALA competes with linoleic acid (LA) metabolism using the same enzyme systems, with the typical Western diet containing approximately one order of magnitude more LA than ALA [145]
Table 1: Key Challenges in ALA Clinical Translation
| Challenge Category | Specific Issues | Impact on Translation |
|---|---|---|
| Metabolic Conversion | Variable conversion to EPA/DHA (0.05-21%) | Difficult to predict clinical efficacy from ALA supplementation |
| Pharmacokinetics | Competitive metabolism with omega-6 fatty acids | Requires careful consideration of background diet in trial design |
| Formulation Stability | Oxidation susceptibility due to bis-allylic hydrogens | Creates manufacturing and shelf-life challenges |
| Biomarker Development | Limited predictive biomarkers for ALA response | Hinders patient stratification and trial efficiency |
| Clinical Trial Design | Need for large, lengthy trials to show chronic disease benefit | Increases costs and complexity of development |
Innovative Strategies and Methodologies
Advanced Modeling and Preclinical Approaches
Improving the predictive validity of preclinical models represents a critical frontier in enhancing translational success. Several innovative approaches show particular promise for ALA research:
Physiologically-Based Pharmacokinetic (PBPK) Modeling: This mathematical modeling technique incorporates physiological parameters, compound-specific properties, and study design elements to predict drug behavior in humans [147]. For ALA research, PBPK modeling could help predict interindividual variability in EPA/DHA conversion based on genetic polymorphisms in desaturase enzymes, hormonal status, and dietary factors.
CRISPR/Cas9-Based Animal Models: Advanced genetic engineering techniques enable creation of more physiologically relevant animal models that better recapitulate human disease pathways [147]. For ALA research, these models could help elucidate the molecular mechanisms underlying its cardioprotective and neuroprotective effects.
Human Tissue Xenograft Models: These models involve implanting human tissue into immunocompromised animals, potentially providing more predictive platforms for studying ALA metabolism and effects in human-relevant systems [143].
Biomarker Development and Precision Medicine Approaches
The integration of biomarkers into therapeutic development represents a powerful strategy for enhancing translational success. Biomarkers can serve multiple functions throughout the drug development continuum:
- Patient stratification: Identifying subpopections most likely to respond to ALA interventions
- Target engagement: Verifying that ALA or its metabolites interact with intended molecular pathways
- Pharmacodynamic assessment: Measuring biological responses to ALA supplementation
- Predictive toxicology: Identifying potential adverse effects earlier in development
For ALA research, promising biomarker approaches include omega-3 index measurements, oxylipin profiling, and inflammatory mediator panels that can provide insight into biological effects beyond simple blood ALA levels [148].
Experimental Design and Methodological Considerations
Dose-Response Meta-Analysis Methodology: Recent meta-analyses on ALA supplementation provide valuable methodological frameworks for future research [139]. Key elements include:
- Systematic search strategy: Comprehensive searching of multiple databases (PubMed, Embase, Scopus, ProQuest) using controlled vocabulary and keywords
- Strict inclusion criteria: Focusing on randomized controlled trials (RCTs) with proper control groups
- Quality assessment: Using Cochrane risk of bias tools and GRADE framework for evaluating evidence quality
- Dose-response evaluation: Assessing effects across different dosage ranges to establish optimal dosing
- Sensitivity analyses: Exploring sources of heterogeneity through subgroup analyses
Clinical Trial Design Considerations: For ALA intervention studies, several design elements require special attention:
- Dietary control: Accounting for background intake of both omega-3 and omega-6 fatty acids
- Run-in periods: Establishing stable baseline nutrient status before intervention
- Compliance assessment: Using both self-report and biochemical validation of compliance
- Appropriate endpoints: Selecting clinically relevant endpoints with sound biological plausibility
ALA Metabolism and Therapeutic Targeting
Metabolic Pathways and Molecular Mechanisms
Alpha-linolenic acid serves as an essential omega-3 fatty acid with multiple physiological roles and health implications. Understanding its complex metabolic pathways is crucial for targeted therapeutic development:
Conversion Pathway: ALA undergoes a series of elongation and desaturation steps to form longer-chain omega-3 PUFAs [145] [146]:
- ALA → stearidonic acid (SDA) via Δ6-desaturase
- SDA → eicosatetraenoic acid (ETA) via elongase
- ETA → eicosapentaenoic acid (EPA) via Δ5-desaturase
- EPA → docosapentaenoic acid (DPA) via elongase
- DPA → docosahexaenoic acid (DHA) via further elongation, desaturation, and β-oxidation
Molecular Targets and Mechanisms: ALA and its metabolites influence multiple physiological processes through various mechanisms:
- Cell membrane incorporation: Affecting membrane fluidity, receptor function, and signal transduction
- Eicosanoid production: Shifting prostaglandin, thromboxane, and leukotriene production toward less inflammatory profiles
- Gene expression regulation: Acting as ligands for transcription factors like PPARs and modulating inflammatory gene expression
- Oxylipin formation: Generating specialized pro-resolving mediators that actively resolve inflammation
Diagram 1: ALA Metabolic Pathway and Targets
Formulation Strategies for ALA-Based Therapeutics
The chemical instability of ALA presents significant formulation challenges that require innovative solutions:
Microencapsulation Technologies: Several microencapsulation methods can protect ALA from oxidation while maintaining bioavailability [145]:
- Spray drying: Most widely used method, parameters include wall materials, oil loading, and temperature control
- Spray chilling: Useful for temperature-sensitive formulations
- Freeze-drying: Preserves structure but is cost-intensive
- Coacervation: Provides excellent controlled release properties
- Extrusion: Creates stable encapsulates for food and pharmaceutical applications
Novel Delivery Systems: Advanced nanomedicine approaches offer promising avenues for ALA delivery [149]:
- Polymer-drug conjugates: Enhancing stability and pharmacokinetic profiles
- Lipid-based nanoparticles: Improving bioavailability and tissue targeting
- Polymeric micelles: Providing controlled release characteristics
- Inorganic nanoparticles: Enabling multimodal therapeutic and diagnostic applications
Table 2: Formulation Strategies for ALA-Based Therapeutics
| Formulation Approach | Key Features | Advantages | Challenges |
|---|---|---|---|
| Microencapsulation (Spray Drying) | Wall materials (proteins, polysaccharides), oil loading 10-50% | Cost-effective, scalable | Potential oxidation during processing |
| Lipid Nanoparticles | Solid lipid or nanostructured lipid carriers | Enhanced bioavailability, targeted delivery | Complex manufacturing, characterization |
| Polymer-Conjugated ALA | Covalent attachment to polymer backbone | Improved pharmacokinetics, reduced oxidation | Altered metabolism, regulatory hurdles |
| Nanoemulsions | Oil-in-water systems with emulsifiers | Improved absorption, versatility | Physical instability, preservative needs |
| Cyclodextrin Complexation | Inclusion complexes | Superior oxidation protection, solubility | Limited loading capacity, cost considerations |
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Research Reagents for ALA and Lipid Metabolism Studies
| Research Tool | Specifications | Research Applications | Key Features |
|---|---|---|---|
| Recombinant Desaturase Enzymes | Human FADS1 (Δ5-desaturase), FADS2 (Δ6-desaturase) | Conversion studies, inhibitor screening | Catalytic activity verification, purity >95% [148] |
| Stable Isotope-Labeled ALA | ¹³C-ALA, deuterated ALA standards | Metabolic tracing, pharmacokinetic studies | Isotopic purity >98%, chemical stability verification |
| Oxylipin Profiling Kits | LC-MS/MS based quantification of 100+ oxylipins | Inflammatory pathway analysis, biomarker discovery | Includes PhytoPs, PhytoFs from non-enzymatic oxidation [145] |
| Fatty Acid Analysis Standards | Deuterated internal standards for EPA, DPA, DHA | Accurate quantification of ALA metabolites | Certified reference materials, purity documentation |
| PPAR Reporter Assay Systems | Cell lines with PPAR-responsive luciferase reporters | Mechanism of action studies | Validated response elements, control constructs |
| Specialized Lipidomics Platforms | Targeted mass spectrometry panels | Comprehensive fatty acid profiling | Quantitative accuracy, wide dynamic range [96] |
| Pre-formed Fibrils (PFFs) | Tau PFFs for neurodegeneration models | AD research, neuroinflammation studies | Induction of protein aggregation verified [148] |
| p-Tau Antibodies | Phospho-specific tau antibodies (p-tau217, p-tau181) | Biomarker assessment, target engagement | Specificity validation, application testing [148] |
Future Perspectives and Concluding Remarks
The future of clinical translation and drug development, particularly in the realm of nutritional biochemistry and natural product-based therapeutics, will be shaped by several emerging trends and technologies. For ALA research specifically, promising directions include:
Precision Nutrition Approaches: The development of biomarkers that predict individual responses to ALA supplementation will enable more targeted and effective interventions. Genetic variants in FADS genes, EPA/ALA conversion efficiency biomarkers, and oxylipin profiling may eventually allow stratification of patients most likely to benefit from ALA-based therapies [148] [145].
Combination Therapy Strategies: Given the complex pathophysiology of chronic diseases, future ALA development may focus on rational combination approaches. These could include ALA with anti-inflammatory agents, ALA with conventional cardiovascular drugs, or ALA with other nutraceuticals having complementary mechanisms of action [148].
Advanced Clinical Trial Designs: Adaptive trial designs, basket trials, and N-of-1 trial methodologies may help overcome some of the challenges inherent in studying natural products with variable metabolism and moderate effect sizes [139].
Integration of Digital Health Technologies: Wearable sensors, mobile health applications, and remote monitoring technologies could enhance the quality and granularity of data collected in ALA intervention trials, potentially revealing benefits that traditional endpoint assessments might miss.
The pathway from basic discovery to clinical application remains challenging, but systematic approaches that address the key bottlenecks in translation—including improved preclinical models, better biomarkers, innovative formulation strategies, and efficient clinical trial designs—offer promise for accelerating the development of ALA-based therapeutics and realizing the full potential of this essential nutrient in clinical medicine.
Conclusion
Alpha-linolenic acid represents a crucial yet complex component of human nutrition with significant therapeutic potential. While ALA demonstrates diverse pharmacological benefits including cardioprotective, anti-inflammatory, and neuroprotective effects, its clinical application is constrained by limited conversion to long-chain metabolites like DHA, particularly in male populations. The metabolic pathway of ALA involves sophisticated cellular coordination between endoplasmic reticulum and peroxisomal compartments, with efficiency influenced by multiple biological and dietary factors. For biomedical researchers and drug development professionals, key challenges remain in optimizing ALA's bioavailability, understanding individual metabolic variability, and developing strategies to enhance its conversion efficiency. Future research should focus on precision nutrition approaches that account for demographic differences, developing novel delivery systems to improve efficacy, and conducting robust clinical trials to establish clear dose-response relationships for specific health conditions. The integration of ALA into targeted therapeutic strategies represents a promising frontier for preventing and managing chronic diseases, particularly metabolic disorders, inflammatory conditions, and certain cancers.
References
- 1. Alpha-Lipoic Acid: Biological Mechanisms and Health Benefits - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC11505271/]
- 2. Therapeutic Potential of Alpha-Lipoic Acid: Unraveling Its Role in Oxidative Stress and Inflammatory Conditions [https://www.mdpi.com/1467-3045/47/5/322]
- 3. Alpha-Lipoic Acid and Glucose Metabolism : A Comprehensive Update... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9824456/]
- 4. Alpha-Lipoic Acid - StatPearls - NCBI Bookshelf [https://www.ncbi.nlm.nih.gov/books/NBK564301/]
- 5. ADME analysis, metabolic prediction, and molecular docking of lipoic acid with SARS-CoV-2 Omicron spike protein | Scientific Reports [https://www.nature.com/articles/s41598-025-93121-2]
- 6. Essential Fatty Acids | Linus Pauling Institute | Oregon State University [https://lpi.oregonstate.edu/mic/other-nutrients/essential-fatty-acids]
- 7. Foods High in Alpha Linolenic Acid ( ALA ) [https://www.myfooddata.com/articles/foods-high-in-ALA.php]
- 8. What impacts the conversion of alpha-linolenic acid to DHA ... [https://examine.com/faq/what-impacts-the-conversion-of-alpha-linolenic-acid-to-dha-and-epa/]
- 9. Effects of Dietary α-Linolenic Acid Treatment and the Efficiency of Its Conversion to Eicosapentaenoic and Docosahexaenoic Acids in Obesity and Related Diseases - PubMed [https://pubmed.ncbi.nlm.nih.gov/35889342/]
- 10. Omega 3-metabolism, absorption, bioavailability and health benefits–A review - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S221343441930088X]
- 11. Immunomodulatory Effects of Omegaâ€3 Fatty Acids: Mechanistic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12087734/]
- 12. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC4404917/]
- 13. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021 - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC8308533/]
- 14. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans - PubMed [https://pubmed.ncbi.nlm.nih.gov/27496755/]
- 15. Validation of a Dietary Questionnaire to Screen Omega-3 Fatty Acids Levels in Healthy Adults - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC6682879/]
- 16. Peroxisomal Stress Response and Inter-Organelle Communication in Cellular Homeostasis and Aging - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC8868334/]
- 17. Reactome | alpha-linolenic acid (ALA) metabolism [https://reactome.org/content/detail/R-HSA-2046106]
- 18. for Protein Transport to Multiple - PMC Pathways Peroxisomes [https://pmc.ncbi.nlm.nih.gov/articles/PMC4726662/]
- 19. take shape | Nature Reviews Molecular Cell Biology Peroxisomes [https://www.nature.com/articles/nrm3700?error=cookies_not_supported&code=a0b9125f-d495-43e1-a6f9-3019d0d1d60d]
- 20. Frontiers | Peroxisome dynamics and inter-organelle interactions in neuronal health and disease [https://www.frontiersin.org/journals/molecular-neuroscience/articles/10.3389/fnmol.2025.1603632/full]
- 21. The effects of concurrent alpha - linolenic , L-carnitine... acid [https://nutritionj.biomedcentral.com/articles/10.1186/s12937-025-01107-7]
- 22. Health benefits of plant-derived α-linolenic acid [https://www.sciencedirect.com/science/article/pii/S0002916523048943]
- 23. FADS1 and FADS2 Polymorphisms Modulate Fatty Acid ... [https://pubmed.ncbi.nlm.nih.gov/31433740/]
- 24. Elongase reactions as control points in long-chain ... [https://pubmed.ncbi.nlm.nih.gov/22216341/]
- 25. FADS1-FADS2 genetic polymorphisms are associated with ... [https://clinicalepigeneticsjournal.biomedcentral.com/articles/10.1186/s13148-018-0545-5]
- 26. A comprehensive review of the family of very-long-chain fatty ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10659008/]
- 27. Functional Characterization of the Chicken Fatty Acid ... [https://www.sciencedirect.com/science/article/pii/S0022316622010859]
- 28. Current Insights into the Effects of Dietary α-Linolenic Acid Focusing on Alterations of Polyunsaturated Fatty Acid Profiles in Metabolic Syndrome - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC11084241/]
- 29. The review of alpha-linolenic acid: Sources, metabolism, and pharmacology - PubMed [https://pubmed.ncbi.nlm.nih.gov/34553434/]
- 30. Metabolism of alpha-linolenic acid in humans - PubMed [https://pubmed.ncbi.nlm.nih.gov/16828546/]
- 31. Quantitation of alpha-linolenic acid elongation to eicosapentaenoic and... [https://nutritionandmetabolism.biomedcentral.com/articles/10.1186/1743-7075-6-8]
- 32. The Biochemistry of Metabolism [https://www.news-medical.net/life-sciences/The-Biochemistry-of-Metabolism.aspx]
- 33. Heme is involved in the exogenous ALA -promoted growth and... [https://bmcplantbiol.biomedcentral.com/articles/10.1186/s12870-022-03717-3]
- 34. Alpha-Lipoic Acid Uses, Benefits & Dosage [https://www.drugs.com/npp/alpha-lipoic-acid.html]
- 35. Alpha-Lipoic Acid in Diabetic Peripheral Neuropathy: Addressing the Challenges and Complexities Surrounding a 70-Year-Old Compound [https://www.mdpi.com/1467-3045/47/6/402]
- 36. Best Alpha Lipoic Acid Supplements | Top 6 in 2025 [https://www.innerbody.com/best-alpha-lipoic-acid-supplements]
- 37. Your Guide To Alpha Lipoic Acid | Swisse Wellness [https://swisse.com.au/wellness-hub/your-guide-to-alpha-lipoic-acid]
- 38. How Alpha Lipoic Acid ALA Works — In One Simple Flow ( 2025 ) [https://www.linkedin.com/pulse/how-alpha-lipoic-acid-ala-works-one-simple-flow-2025-metricsflow-c93if]
- 39. What is Alpha Lipoic Acid Supplements? Uses, How It Works & Top... [https://www.linkedin.com/pulse/what-alpha-lipoic-acid-supplements-uses-how-works-top-qa8uc]
- 40. labeling - Wikipedia Isotopic [https://en.wikipedia.org/wiki/Isotopic_labeling]
- 41. Applications of stable , nonradioactive isotope in in vivo human... tracers [https://www.nature.com/articles/emm201597?error=cookies_not_supported&code=4a5015b0-2160-4fc6-8cbc-d1cf8fbbe92c]
- 42. Exploring Stable in Protein Isotope Tracers Metabolism [https://www.technologynetworks.com/analysis/articles/the-role-of-stable-isotope-tracers-in-skeletal-muscle-metabolism-research-396680]
- 43. Dietary intake and biomarkers of alpha and risk of all... linolenic acid [https://pmc.ncbi.nlm.nih.gov/articles/PMC8513503/]
- 44. In-vivo measurement of glucose and alanine metabolism with stable ... [https://www.scilit.com/publications/601ea41de87fe9e7e62231701f0bf3f1]
- 45. Isotope-ratio mass spectrometry - Wikipedia [https://en.wikipedia.org/wiki/Isotope-ratio_mass_spectrometry]
- 46. A timeline of stable isotopes and mass spectrometry in the life sciences - PubMed [https://pubmed.ncbi.nlm.nih.gov/26919394/]
- 47. Alpha-Linolenic Acid: An Omega-3 Fatty Acid with Neuroprotective Properties—Ready for Use in the Stroke Clinic? - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC4350958/]
- 48. Alpha-linolenic Acid: Health Benefits, Side Effects, Uses, Dose & Precautions [https://www.rxlist.com/supplements/alpha-linolenic_acid.htm]
- 49. Health benefits of plant-derived α-linolenic acid - PubMed [https://pubmed.ncbi.nlm.nih.gov/24898228/]
- 50. Dietary alpha-linolenic acid and health-related outcomes: a metabolic perspective - PubMed [https://pubmed.ncbi.nlm.nih.gov/19079874/]
- 51. Effects of meal frequency on metabolic profiles and substrate... [https://pubmed.ncbi.nlm.nih.gov/22719910/]
- 52. Guide to Metabolomics Analysis: A Bioinformatics Workflow - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC9032224/]
- 53. KEGG PATHWAY Database [https://www.genome.jp/kegg/pathway.html]
- 54. MetaboAnalyst [https://www.metaboanalyst.ca/]
- 55. MetaCyc: Metabolic Pathways From all Domains of Life [https://metacyc.org/]
- 56. The Role of α- Linolenic and Its Oxylipins in Human... Acid [https://pmc.ncbi.nlm.nih.gov/articles/PMC10093787/]
- 57. : Plant-Based Omega-3 Supports Heart, Cognition - Dr. Axe ALA [https://draxe.com/health/alpha-linolenic-acid-plant-based-omega-3-supports-heart-cognition/]
- 58. 15-Lipoxygenase metabolites of α-linolenic acid, [13-(S)-HPOTrE and...] [https://www.nature.com/articles/srep31649?error=cookies_not_supported&code=7dea5f10-13fc-4078-a153-60ca5d8f289d]
- 59. Visualization of metabolites and microbes at high spatial resolution using MALDI mass spectrometry imaging and in situ fluorescence labeling | Nature Protocols [https://www.nature.com/articles/s41596-023-00864-1]
- 60. Correlation between gut bacteria Phascolarctobacterium and... [https://atm.amegroups.org/article/view/101498/html]
- 61. A High-Throughput Targeted Metabolomics Workflow for the Detection of 200 Polar Metabolites in Central Carbon Metabolism - PubMed [https://pubmed.ncbi.nlm.nih.gov/30421235/]
- 62. Gut microbiota-related metabolite - alpha mitigates... linolenic acid [https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-023-01681-0]
- 63. Alpha-Linolenic Acid and Cardiovascular Events: A Narrative Review - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC10531611/]
- 64. Circulating fatty acid profiles impact total, cardiovascular disease, and cancer mortality in a population-based prospective cohort study - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S0261561425000354]
- 65. Alpha-linolenic acid and cardiovascular diseases - PubMed [https://pubmed.ncbi.nlm.nih.gov/11458289/]
- 66. Genome-wide characterization of circulating metabolic biomarkers | Nature [https://www.nature.com/articles/s41586-024-07148-y]
- 67. Forecasting of potential anti - inflammatory targets of some... [https://www.nature.com/articles/s41598-023-36540-3?error=cookies_not_supported&code=17b36565-47e2-4521-bc7b-23ad5b525a04]
- 68. Alpha-lipoic acid: mechanisms of action and... - MedCrave online [https://medcraveonline.com/MOJPH/alpha-lipoic-acid-mechanisms-of-action-and-beneficial-effects-in-the-prevention-and-treatment-of-diabetic-complications.html]
- 69. activities of alpha lipoic acid with a special focus... Immunomodulatory [https://pubmed.ncbi.nlm.nih.gov/27292272/]
- 70. - Anti and inflammatory of... immunomodulatory mechanisms [https://jneuroinflammation.biomedcentral.com/articles/10.1186/1742-2094-10-106]
- 71. A versatile toolkit for drug metabolism studies with GNPS2: from drug development to clinical monitoring | Nature Protocols [https://www.nature.com/articles/s41596-025-01237-6]
- 72. Alzheimer's disease and Parkinson's disease: distinct entities or extremes of a spectrum of neurodegeneration? - PubMed [https://pubmed.ncbi.nlm.nih.gov/9749570/]
- 73. Disease Connections: Alzheimer’s and Parkinson’s | American Brain Foundation [https://www.americanbrainfoundation.org/disease-connections-alzheimers-and-parkinsons/]
- 74. of Neuroprotective - Effect against Aβ-Mediated... Alpha Linolenic Acid [https://pubmed.ncbi.nlm.nih.gov/29668263/]
- 75. Oral consumption of α- linolenic increases serum BDNF levels in... acid [https://nutritionj.biomedcentral.com/articles/10.1186/s12937-015-0012-5]
- 76. Alzheimer's disease drug development pipeline: 2025 - PubMed [https://pubmed.ncbi.nlm.nih.gov/40463637/]
- 77. The Multifaceted Role of Alpha-Lipoic Acid in Cancer ... Prevention [https://pmc.ncbi.nlm.nih.gov/articles/PMC11351715/]
- 78. New Advances in Metabolic Syndrome, from Prevention to Treatment: The Role of Diet and Food - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC9921449/]
- 79. Frontiers | Metabolic syndrome: epidemiology, mechanisms, and current therapeutic approaches [https://www.frontiersin.org/journals/nutrition/articles/10.3389/fnut.2025.1661603/full]
- 80. of Conversion to Efficiency in Humans | ALA /EPE... DHA DHA [https://dhaomega3.org/overview/conversion-efficiency-of-ala-to-dha-in-humans]
- 81. Are all n-3 polyunsaturated fatty acids created equal? - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC3224740/]
- 82. Biosynthesis of docosahexaenoic acid (DHA, 22:6-4, 7,10,13,16,19): two distinct pathways - PubMed [https://pubmed.ncbi.nlm.nih.gov/12538082/]
- 83. PUFA synthase-independent DHA synthesis pathway in Parietichytrium sp. and its modification to produce EPA and n-3DPA | Communications Biology [https://www.nature.com/articles/s42003-021-02857-w]
- 84. Effects of a 12-week high-α- linolenic intervention on EPA and... acid [https://pubs.rsc.org/en/content/articlehtml/2018/fo/c7fo01809f]
- 85. Fucoxanthin promotes the conversion of efficiency - alpha ... linolenic [https://pubmed.ncbi.nlm.nih.gov/39824082/]
- 86. in the n-3 fatty Gender content of tissues differences acid [https://pubmed.ncbi.nlm.nih.gov/18234128/]
- 87. Influence of gender on the pharmacokinetics and pharmacodynamics of drugs - PubMed [https://pubmed.ncbi.nlm.nih.gov/9849747/]
- 88. Resting metabolic rate is lower in women than in men - PubMed [https://pubmed.ncbi.nlm.nih.gov/8125870/]
- 89. Gender Differences in Metabolism – An Ecological Approach to Obesity and Eating Disorders [https://pressbooks.pub/btugman/chapter/gender-differences-in-metabolism/]
- 90. How Gender Affects Metabolism and Nutrition - Simple Science [https://scisimple.com/en/articles/2025-10-16-how-gender-affects-metabolism-and-nutrition--a3oyz4w]
- 91. Gender differences in drug metabolism regulated by growth hormone - ScienceDirect [https://www.sciencedirect.com/science/article/pii/1357272594000565]
- 92. sciencedirect.com/science/article/abs/pii/S1440244000800389 [https://www.sciencedirect.com/science/article/abs/pii/S1440244000800389]
- 93. Regulation of drug-metabolizing enzymes by sex-related hormones: clinical implications for transgender medicine - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S0165614722000566]
- 94. Integrated metabolomics and transcriptomics analysis comprehensively... [https://bmcplantbiol.biomedcentral.com/articles/10.1186/s12870-025-06575-x]
- 95. Impact of α-Linolenic Acid, the Vegetable ω-3 Fatty Acid, on Cardiovascular Disease and Cognition - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC9526859/]
- 96. Alpha-Linolenic Acid | Rupa Health [https://www.rupahealth.com/biomarkers/alpha-linolenic-acid]
- 97. Dietary flaxseed: Cardiometabolic benefits and its role in promoting... [https://pubmed.ncbi.nlm.nih.gov/39821819/]
- 98. Systems biology modelling based evaluation of omega-3 formulation in managing cardiovascular and cerebrovascular risk | Scientific Reports [https://www.nature.com/articles/s41598-025-23677-6]
- 99. Dose-response characterization: A key to success in drug development - PubMed [https://pubmed.ncbi.nlm.nih.gov/40626332/]
- 100. Comparison of the metabolism of linoleic and linolenic ... [https://pubmed.ncbi.nlm.nih.gov/2892461/]
- 101. The Effects of α-Linolenic Acid Intake on Skin and Blood ... [https://www.sciencedirect.com/science/article/pii/S0022316625004754]
- 102. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC9495787/]
- 103. Chemical approaches for the enhancement of 5-aminolevulinic... [https://pubs.rsc.org/en/content/articlehtml/2018/pp/c8pp00362a]
- 104. Metabolism of α-linolenic acid in humans - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S0952327806000834]
- 105. Current Insights into the Effects of Dietary α-Linolenic Acid Focusing on Alterations of Polyunsaturated Fatty Acid Profiles in Metabolic Syndrome [https://www.mdpi.com/1422-0067/25/9/4909]
- 106. Fluxomics - New Metabolomics Approaches to Monitor Metabolic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8977530/]
- 107. (PDF) Dietary α- linolenic and acid -related outcomes... health [https://www.academia.edu/15820408/Dietary_%CE%B1_linolenic_acid_and_health_related_outcomes_a_metabolic_perspective]
- 108. analysis for metabolome data validation of naturally... Metabolic flux [https://bmcbiotechnol.biomedcentral.com/articles/10.1186/s12896-019-0548-0]
- 109. What is flux balance analysis? - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC3108565/]
- 110. Advances in flux balance analysis by integrating machine learning and mechanism-based models - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC8382995/]
- 111. reveals the... | Nature Microbiology Metabolic modelling [https://www.nature.com/articles/s41564-025-01959-z?error=cookies_not_supported&code=649e58a7-b243-4377-823f-b98c1a5643bb]
- 112. - Alpha ( Linolenic ) -Nutritional Modulation of the... :: SSRN Acid ALA [https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5535839]
- 113. Alpha-lipoic acid alleviates cognitive deficits in transgenic APP23/PS45... [https://alzres.biomedcentral.com/articles/10.1186/s13195-024-01527-3]
- 114. Alpha-lipoic acid (ALA) supplementation effect on glycemic and inflammatory biomarkers: A Systematic Review and meta- analysis - PubMed [https://pubmed.ncbi.nlm.nih.gov/31221283/]
- 115. Efficacy and safety of oral alpha-lipoic acid supplementation for type 2 diabetes management: a systematic review and dose-response meta-analysis of randomized trials - PubMed [https://pubmed.ncbi.nlm.nih.gov/36006850/]
- 116. Bioavailability as Proof to Authorize the Clinical Testing of Neurodegenerative Drugs—Protocols and Advice for the FDA to Meet the ALS Act Vision - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC11432374/]
- 117. . Plant Marine - vs s: Key Differences – MASI Longevity Science Omega 3 [https://masi.eu/en-aud/blogs/longevity-news/marine-vs-plant-omega-3s-key-differences]
- 118. Differentiation of ALA (plant sources) from DHA + EPA ( marine ... [https://dhaomega3.org/overview/differentiation-of-ala-plant-sources-from-dha-epa-marine-sources-as-dietary-omega-3-fatty-acids-for-human-health]
- 119. Bioavailability of EPA and DHA in humans - A comprehensive review - PubMed [https://pubmed.ncbi.nlm.nih.gov/39736417/]
- 120. - Omega Supplements: What You Need To Know | NCCIH 3 [https://www.nccih.nih.gov/health/omega3-supplements-what-you-need-to-know]
- 121. - Omega . Omega-6: Key Differences Between... | mindbodygreen 3 vs [https://www.mindbodygreen.com/articles/omega-3-vs-omega-6]
- 122. Frontiers | Association of Dietary Intake and Biomarker of α- Linolenic ... [https://www.frontiersin.org/journals/nutrition/articles/10.3389/fnut.2022.948604/full]
- 123. in Targeted Metabolomics Validating Biomarkers [https://www.news-medical.net/life-sciences/Validating-Biomarkers-in-Targeted-Metabolomics.aspx]
- 124. ELISA - Wikipedia [https://en.wikipedia.org/wiki/ELISA]
- 125. Overview of ELISA | Thermo Fisher Scientific - US [https://www.thermofisher.com/us/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/overview-elisa.html]
- 126. The Antitumor Effects of α-Linolenic Acid - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC10971543/]
- 127. Alpha-Lipoic Acid: Biological Mechanisms and Health ... [https://www.mdpi.com/2076-3921/13/10/1228]
- 128. Identification of the alpha linolenic acid metabolism-related ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9388792/]
- 129. α-lipoic acid modulates prostate cancer cell growth and ... [https://www.nature.com/articles/s41598-024-54479-x]
- 130. Alpha lipoic acid as a modulator of the AMPK-p53 axis [https://accscience.com/journal/CP/6/4/10.36922/cp.4867]
- 131. Alpha Lipoic Acid and Cancer [https://www.cancertherapyadvisor.com/factsheets/alpha-lipoic-acid-and-cancer/]
- 132. Emerging targets in lipid metabolism for cancer therapy - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC11162322/]
- 133. Biotechnological production of omega-3 fatty acids: current status and future perspectives - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC10662050/]
- 134. Dietary intake and biomarkers of alpha linolenic acid and risk of all cause, cardiovascular, and cancer mortality: systematic review and dose-response meta-analysis of cohort studies - PubMed [https://pubmed.ncbi.nlm.nih.gov/34645650/]
- 135. Producing Omega-3 Polyunsaturated Fatty Acids: A Review of Sustainable Sources and Future Trends for the EPA and DHA Market | MDPI [https://www.mdpi.com/2079-9276/9/12/148]
- 136. Algae Omega 3 Market - Market Size, Sustainable Insights and Growth Report 2024-2031 [https://www.datamintelligence.com/research-report/algae-omega-3-market]
- 137. Long-Chain Omega-3 Oils–An Update on Sustainable Sources - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC3257669/]
- 138. Alpha Lipoic Acid Benefits, Sources, Dosage and Side Effects - Dr. Axe [https://draxe.com/nutrition/alpha-lipoic-acid/]
- 139. Frontiers | The effects of alpha lipoic acid ( ALA ) supplementation on... [https://www.frontiersin.org/journals/cardiovascular-medicine/articles/10.3389/fcvm.2023.1272837/full]
- 140. Alpha Lipoic Acid Benefits and Side Effects [https://www.webmd.com/diet/alpha-lipoic-acid-ala]
- 141. Effects of alpha-lipoic acid supplementation on cardiometabolic risk ... [https://pubmed.ncbi.nlm.nih.gov/41077538/]
- 142. Alpha-Lipoic Acid benefits, dosage, and side effects [https://examine.com/supplements/alpha-lipoic-acid/]
- 143. Lost in translation: the valley of death across preclinical and clinical divide – identification of problems and overcoming obstacles | Translational Medicine Communications | Full Text [https://transmedcomms.biomedcentral.com/articles/10.1186/s41231-019-0050-7]
- 144. Translational research: current status, challenges and future strategies - PubMed [https://pubmed.ncbi.nlm.nih.gov/22046484/]
- 145. sciencedirect.com/topics/food-science/ alpha - linolenic - acid [https://www.sciencedirect.com/topics/food-science/alpha-linolenic-acid]
- 146. α- Linolenic - Wikipedia acid [https://en.wikipedia.org/wiki/%CE%91-Linolenic_acid]
- 147. Current trends in drug and pharmacokinetics metabolism [https://pubmed.ncbi.nlm.nih.gov/31867160/]
- 148. 文献解读 | 2025阿尔茨海默病è¯ç‰©ç ”å‘管线全景 | ACROBiosystems百普赛斯生物科技股份有é™å…¬å¸ [https://www.acrobiosystems.cn/insights/5706]
- 149. Challenges in nanomedicine clinical translation | Drug Delivery and Translational Research [https://link.springer.com/article/10.1007/s13346-020-00740-5]